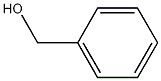
Benzyl alcohol (CAS 100-51-6) is a clear, colorless liquid with a mild, pleasant odor. It has a basic structure consisting of a benzene ring with a hydroxyl group (-OH) attached. This chemical is highly soluble in water, meaning it can easily dissolve in it. It also exhibits moderate solubility in organic solvents such as ethanol and ether. Benzyl alcohol has a boiling point of 205°C and a melting point of -15°C.
Benzyl alcohol possesses various physical and chemical properties. It is flammable and can form explosive mixtures with air. It is also a volatile compound, meaning it can evaporate easily. Benzyl alcohol is stable under normal conditions but may react with strong oxidizing agents. It can undergo oxidation to form benzaldehyde or benzoic acid. Additionally, it can react with acids to form esters.
Applicable fields
Benzyl alcohol finds application in several industries due to its properties. It is commonly used in the pharmaceutical industry as a solvent and preservative. It can help dissolve and stabilize various active ingredients in medications. In the personal care industry, benzyl alcohol is used as a fragrance ingredient and a solvent in cosmetic formulations. It can also act as a preservative, preventing the growth of microorganisms in products.
Mechanism of action
In pharmaceutical applications, benzyl alcohol acts as a solvent, allowing the dissolution of hydrophobic drugs. It can also function as a preservative by inhibiting the growth of bacteria and fungi. In the personal care industry, benzyl alcohol provides a pleasant scent to products and helps dissolve other fragrance ingredients. Its preservative properties help extend the shelf life of cosmetic formulations.
Storage Conditions
Store in a cool, dry place.