Step A: Hydrogen sulfide gas (HStep A: Step A: Hydrogen sulfide gas (H2S) was bubbled into a solution of ethyl cyanoacetate (4.7 mL, 44.3 mmol) in pyridine/triethylamine (500 mL, 1 : 1 v/v) until it became saturated. The mixture was heated at 60 C for 18 h, then the solvent was removed under vacuum. The residue was partitioned between EtOAc and aqueous HC1. The organic layer was dried over MgS04, then filtered and concentrated under vacuum. The resulting oil was filtered to remove solid impurities. Ethyl 3-amino-3-thioxopropanoate (6.25 g, 96%) was obtained as an orange oil. 1H NMR (500 MHz, CDC13): delta 8.92 (1H, br s), 7.75 (1H, br s), 4.21 (2H, q, J= 7 Hz), 3.82 (2H, s), 1.29 (3H, t, J= 7 Hz).Step A: Hydrogen sulfide gas (H2S) was bubbled into a solution of ethyl cyanoacetate (4.7 mL, 44.3 mmol) in pyridine/triethylamine (500 mL, 1:1 v/v) until it became saturated. The mixture was heated at 60 C. for 18 h, then the solvent was removed under vacuum. The residue was partitioned between EtOAc and aqueous HCl. The organic layer was dried over MgSO4, then filtered and concentrated under vacuum. The resulting oil was filtered to remove solid impurities. Ethyl 3-amino-3-thioxopropanoate (6.25 g, 96%) was obtained as an orange oil. 1H NMR (500 MHz, CDCl3): delta 8.92 (1H, br s), 7.75 (1H, br s), 4.21 (2H, q, J=7 Hz), 3.82 (2H, s), 1.29 (3H, t, J=7 Hz).Step B: A solution of Step B: A solution of ethyl 3-amino-3-thioxopropanate Oil 1H-NMR (CDCl3) delta: 1.31 (3H, t, J=7.1 Hz), 3.85 (2H, s), 4.22 (2H, q, J=7.1 Hz), 7.74 (1H, br s), 8.92 (1H, br s).To a mixture of 2-1 (50 mg, 0.17 mmol, 1 eq) in toluene (3 mL) and AcOH (0.3 mL) was added 2-2 (26.2 mg, 0.18 mmol, 1.05 eq). The resulted mixture was stirred at 90 C for 17 h.LCMS showed the starting material was consumed completed, and 9% desired compound was detected. The mixture was stirred at 100 C for 3 h. LCMS showed there's no obvious change. The mixture was concentrated in vacuum. The residue was checked by HPLC. The residue was purified by prep-HPLC. 2-3 (10 mg, 25.6 umol, 15.1% yield) was obtained.
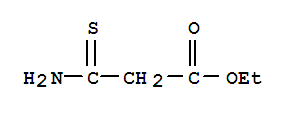
|

|