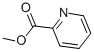
Methyl picolinate, with the chemical formula C8H7NO2, has the CAS number 2459-07-6. It appears as a colorless to pale yellow liquid with a fruity odor. The basic structure of methyl picolinate consists of a methyl group attached to a pyridine ring. This compound is soluble in organic solvents but insoluble in water. Methyl picolinate should be handled with care as it may cause irritation to the skin, eyes, and respiratory system. It is recommended to use appropriate protective equipment when working with this chemical. In case of ingestion or inhalation, seek medical attention immediately. Methyl picolinate is not classified as a hazardous substance, but it should be stored and handled in a well-ventilated area to avoid exposure.
Applicable Fields
Organic Synthesis: Methyl picolinate is commonly used as a building block in organic synthesis. Its purpose in this field involves its ability to react with other compounds to form more complex molecules. The mechanism of action in organic synthesis relies on the reactivity of the pyridine ring and the methyl group, which can undergo various chemical transformations.
Pharmaceuticals: Methyl picolinate has potential applications in the pharmaceutical industry. Its purpose in this field involves its ability to act as a ligand for metal ions or as a precursor for the synthesis of biologically active compounds. The mechanism of action in pharmaceuticals can vary depending on the specific application, but it often involves the interaction of methyl picolinate with target molecules or enzymes.
Storage
Conditions: Store in a cool and dry place.
|

|

|

|

|

|