Hydroxylamine hydrochloride, with the chemical formula NH2OH·HCl, has the CAS number 5470-11-1. It appears as a white crystalline solid with a strong ammonia-like odor. The basic structure of hydroxylamine hydrochloride consists of a hydroxylamine molecule bonded to a hydrogen chloride molecule. This compound is highly soluble in water. Hydroxylamine hydrochloride is a strong reducing agent and can react violently with oxidizing agents. It is also corrosive to metals and may cause severe skin burns and eye damage. Ingestion or inhalation of hydroxylamine hydrochloride can cause serious health effects and should be avoided. It is important to handle this chemical with caution and use appropriate protective measures.
Applicable Fields
Chemical synthesis: Hydroxylamine hydrochloride is commonly used in chemical synthesis as a reducing agent. Its purpose in this field involves its ability to donate electrons and facilitate the reduction of other compounds. The mechanism of action in chemical synthesis involves the transfer of electrons from hydroxylamine hydrochloride to the target compound, resulting in a reduction reaction.
Photography: This compound is also used in the field of photography. Its purpose in this context involves its ability to act as a developer, converting exposed silver halide crystals into metallic silver. The mechanism of action in photography involves the reduction of silver ions by hydroxylamine hydrochloride, leading to the formation of metallic silver.
Storage
Conditions: Store in a cool and dry place.

|

|

|
 |
 |
 |
 |
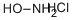
|

|

|

|

|

|

|

|
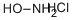
|

|

|

|

|

|