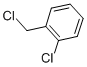
2-Chlorobenzyl chloride, with the chemical formula C7H6Cl2, has the CAS number 611-19-8. It appears as a colorless to pale yellow liquid with a pungent odor. The basic structure of 2-Chlorobenzyl chloride consists of a benzene ring with a chlorine atom and a benzyl group attached to it. This compound is insoluble in water. 2-Chlorobenzyl chloride is a hazardous substance and should be handled with care. It may cause irritation to the skin, eyes, and respiratory system. Ingestion or inhalation of this chemical can lead to serious health effects. It is important to use appropriate protective measures when working with 2-Chlorobenzyl chloride, such as wearing gloves and a respirator. Proper ventilation is also necessary to prevent the accumulation of vapors in the air. In case of a spill, it is important to contain and clean up the chemical properly to prevent further exposure and environmental contamination.
Applicable Fields
Pharmaceuticals: 2-Chlorobenzyl chloride is commonly used as a building block in the synthesis of various pharmaceutical compounds. Its purpose in this field involves its ability to introduce the benzyl chloride functional group, which is often used as a reactive moiety in drug synthesis. The mechanism of action in pharmaceuticals involves the reaction of 2-Chlorobenzyl chloride with other reagents to form desired pharmaceutical intermediates or final products.
Chemical synthesis: This compound is also utilized in various chemical synthesis processes. Its purpose in this field involves its reactivity as an electrophile, allowing it to undergo substitution reactions with nucleophiles. The mechanism of action in chemical synthesis involves the reaction of 2-Chlorobenzyl chloride with other compounds to form new chemical entities or functional groups.
Storage
Conditions: Store in a cool and dry place.

|

|

|

|

|

|

|
|

|

|

|

|

|

|