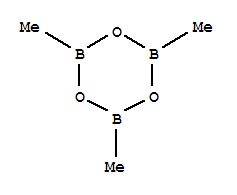
Trimethylboroxine, with the chemical formula C3H9BO3, has the CAS number 823-96-1. It appears as a colorless liquid with a mild odor. The basic structure of trimethylboroxine consists of three methyl groups attached to a boron atom, which is in turn bonded to three oxygen atoms. This compound is soluble in water. Trimethylboroxine is considered to be relatively safe for use, with no significant safety concerns reported. However, as with any chemical, it is important to handle it with care and follow proper safety precautions when working with it.
Applicable Fields
Chemical synthesis: Trimethylboroxine is commonly used as a reagent in organic synthesis reactions. Its purpose in this field involves its ability to act as a boron source, which can be utilized in various reactions such as boronate ester formation and boron-mediated transformations. The mechanism of action in chemical synthesis involves the interaction of trimethylboroxine with other reagents to facilitate the desired chemical transformations.
Flame retardants: This compound has also found applications as a flame retardant. Its purpose in this field involves its ability to inhibit or delay the spread of flames in materials. The mechanism of action in flame retardants is typically based on the release of non-combustible gases or the formation of a protective char layer when exposed to heat or flames.
Storage
Conditions: Store in a cool and dry place.

|

|

|
|

|

|

|

|

|