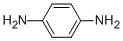
CAS 106-50-3 p-Phenylenediamine | Products & Prices & Suppliersts
It is white to purple red crystal. It will turn purple red or dark brown when exposure to the air. It can be dissolved in water, alcohol, ether, chloroform and benzene. p-Phenylenediamines are white to slightly red crystalline solids. They have been described as gray “light brown” which may result from exposure to air.ChEBI: A phenylenediamine in which the amino functions are at positions 1 and 4 of the benzene nucleus.P-phenylenediamine is one of the simplest aromatic diamine with the pure p
ldentification
- Cas NO.: 106-50-3
- CID: 7814
- Mw: 108.14112
- MF: C6H8N2
- Name:p-Phenylenediamine (Related Reference)
- Synonyms: 1,4-BENZENEDIAMINE; 1,4-DIAMINOBENZENE; 1,4-phenyldiamine; 1,4-PHENYLENEDIAMINE; 4-AMINOANILINE; BENZENE-1,4-DIAMINE; C.I. 76060; EINECS 203-404-7; fur black 41866; JAROCOL PPD; MFCD00007901; paraphenylenediamine;
- EINECS: 203-404-7
Safety Data
- [Risk Codes]
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed
R36:Irritating to the eyes
R43:May cause sensitization by skin contact
R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment
- [Safety Statements]
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer)
S36/37:Wear suitable protective clothing and gloves
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
S60:This material and/or its container must be disposed of as hazardous waste
S61:Avoid release to the environment. Refer to special instructions safety data sheet
- Hazard Symbols:
HazardClass: 6.1
Properties
-
- Appearance:white to light purple solid
- Molecular Weight:108.14112
- Density:1.15
- Boiling Point:267℃
- Melting Point:138-143℃
- Flash Point:156℃
- Storage Temperature:Store below +30°C.
- Refractive index:1.661
- Solubility:47 g/L (25 oC)
- Stability:Stable, but oxidizes when exposed to air. Incompatible with oxidizing agents. Store under inert atmosphere.
- More Properties>>