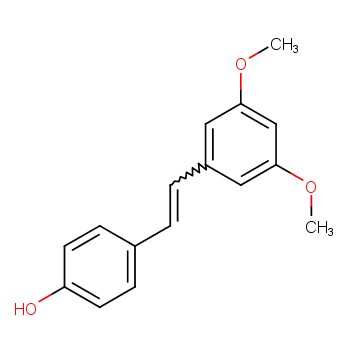
[Overview]
Pterostilbene (E-3,5-dimethoxy-4,-hydroxystilbene), also called Pterostilbene, is derived from blue strawberry, Indian gina tree and sword-leaf dracaena in Guangxi, China A trans-stilbene compound obtained from natural plants, it has multiple effects such as anti-cancer, antioxidant, hypoglycemic, COX-1 and COX-2 inhibitory and anti-fungal effects. It is the main active ingredient in Ayuvedic, a traditional medicine for diabetes in Ayurvedic medicine. It has the activity of activating peroxisome proliferator-activated receptors and can reduce the blood lipid and blood sugar levels in animals. It is also a chemopreventive agent for cancer. Intake of pterostilbene in the diet is beneficial to health. It has certain effects on the treatment of cancer, hypertension, and hyperlipidemia.
[Physical and Chemical Properties]
Density: 1.169 g/cm3 Boiling point: 420.4℃ at 760 mmHg Melting point: 89-92℃ Flash point: 208.1℃ Refractive index: 1.639 Vapor pressure: 1.15E-07mmHg at 25℃ Appearance and properties: Off-white crystalline powder
p>
[Preparation method]
1. A method of synthesizing rosewood stilbene
1. Preparation of stilbene intermediates
(1) To prepare the intermediate 3,5-dimethoxybenzyl bromide, take 3,5-dimethoxybenzyl alcohol and HBr solution with a concentration greater than 40%, 3,5-dimethoxybenzyl alcohol and HBr respectively. The dosage of the solution is 1:5-10 in terms of g: ml, and the reaction is carried out at 50-100°C for 10-28 hours to obtain 3,5-dimethoxybenzyl bromide; the preferred concentration is 48%;
(2) Preparation of intermediate 4, ?0?tetrahydropyranbenzaldehyde 1,4?dihydropyran and p-hydroxybenzaldehyde are dissolved in the solvent and reacted at 20~45℃ for 1~5h, the reaction is completed Afterwards, alkali solution is added for treatment, and the organic phase is recovered to obtain 4,0 tetrahydropyranyl benzaldehyde; an acidic catalyst should be added during the reaction, in which the input amount of 3,5 dimethoxybenzyl bromide and p-hydroxybenzaldehyde solution is in moles: mol is calculated as 1:1~2.5; the solvent is THF, dichloromethane or dichloroethane;
2. Preparation of 3,5 dialkyl 4,O tetrahydropyran stilbene. The prepared 3,5 dimethoxy benzyl bromide and trimethyl phosphite are refluxed to obtain 3,5 dimethoxy benzene. Dimethyl methyl phosphonate is then mixed with 4,0 tetrahydropyranyl benzaldehyde in the reaction solvent, and reacted at 0°C to 40°C for 2h to 10h to obtain 3,5dimethoxy 4,O Tetrahydropyran stilbene; the reaction solvent is DMF or THF;
3. Preparation of pterostilbene. Place the prepared 3,5-methoxy 4,0 tetrahydropyran stilbene in the reaction solvent, then add an acidic catalyst, and deprotect the group under the catalysis of the acidic catalyst to obtain the product. For rosewood stilbene, after the reaction is completed, the solvent is recovered under reduced pressure. After treatment, the finished rosewood stilbene is obtained. The reaction temperature is 25°C to 80°C; the reaction solvent is methanol or ethanol, and the reaction solvent is mixed with 3,5 methoxy 4,0 The feeding amount of hydropyran stilbene is 5 to 10:1 in terms of g:ml, and the reaction time is 1h to 6h.

Figure 1 shows the synthetic route 1 of pterostilbene
Intermediate (E)-3,5-dimethoxy is prepared by Grignard reaction of 2, 3,5-dimethoxybenzyl chloride and p-benzyloxybenzaldehyde and catalytic dehydration of solid acid catalyst NaHSO4.SiO2 -4-benzyloxystilbene, and then catalyzed hydrogenation and debenzylation by Pd/Al-MCM-41 mesoporous molecular sieve to prepare pterostilbene.

Figure 2 shows the synthetic route 2 of pterostilbene
[Pharmacological effects]
Antioxidants, active antioxidants with anti-inflammatory, anti-thrombotic, anti-cancer, anti-cancer, anti-hyperlipidemia, antibacterial and other aspects, anti-cell proliferation, lowering blood lipids, inhibiting COX-1 and COX-2, anti- Cancer, antifungal
[Application]
Pterostilbene helps stop pre-cancerous damage to the body. Acts as an antioxidant and anti-inflammatory agent, found in both blueberries and blackberries. The Ohio State University found that animals that eat brownberries have an 80% lower risk of colon cancer and a 60% lower risk of digestive tract tumors. Colon cancer is one of the common malignant tumors, with the highest incidence rate in the 40 to 50-year-old age group. According to a world epidemiological survey, it was found that colon cancer has the highest incidence rate in North America, Western Europe, Australia, New Zealand and other places, ranking second among visceral tumors. The incidence and mortality rates in my country are increasing year by year. After years of experiments on animals, American scientists have discovered that this compound extracted from blueberries and grapes can inhibit an enzyme called "P450 cytochrome" that activates chemical carcinogens and can reduce low-density lipoprotein cholesterol. Therefore, it has the function of preventing cancer and heart disease. Pterostilbene is a fragrant hydrocarbon called "styrene", which is a derivative of resveratrol. In the early 1990s, American scientists discovered that resveratrol has the ability to prevent breast cancer and heart disease, and is found in large amounts in red grape skins.
【Safety Instructions】
After accidental contact with eyes, please rinse immediately with plenty of water and seek medical advice. Wear safety goggles or a mask. Avoid release into the environment. Refer to Special Instructions/Safety Data Sheet. Serious damage to eyes. It is toxic to aquatic life and may have long-term adverse effects on the water environment.