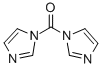
1,1'-Carbonyldiimidazole, with the chemical formula C9H6N4O and CAS registry number 530-62-1, is a compound known for its applications in organic synthesis. This white crystalline solid is characterized by its carbonyl and imidazole functional groups. It is commonly used as a coupling reagent in peptide synthesis, facilitating the formation of peptide bonds between amino acids. Additionally, 1,1'-Carbonyldiimidazole is utilized in the synthesis of various heterocyclic compounds and as a catalyst in organic reactions. Its unique structure and reactivity make it a valuable tool in the field of chemical research and drug discovery.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. Safety and Handling
5. MSDS
6. NMR Spectrum
7. Synthesis Route
8. Precursor and Product
9. Computed Properties
12. Related Questions
13. Realated Product Infomation

 EN
EN

 C,
C, Xi
Xi