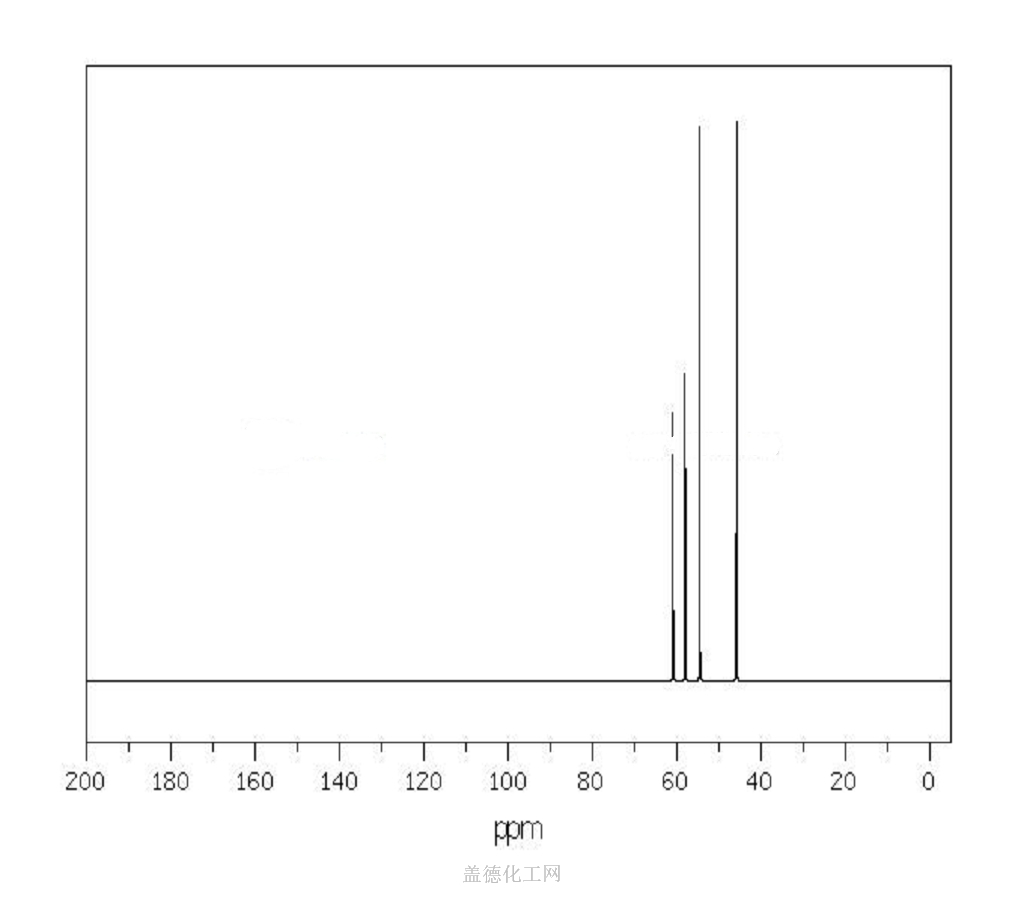
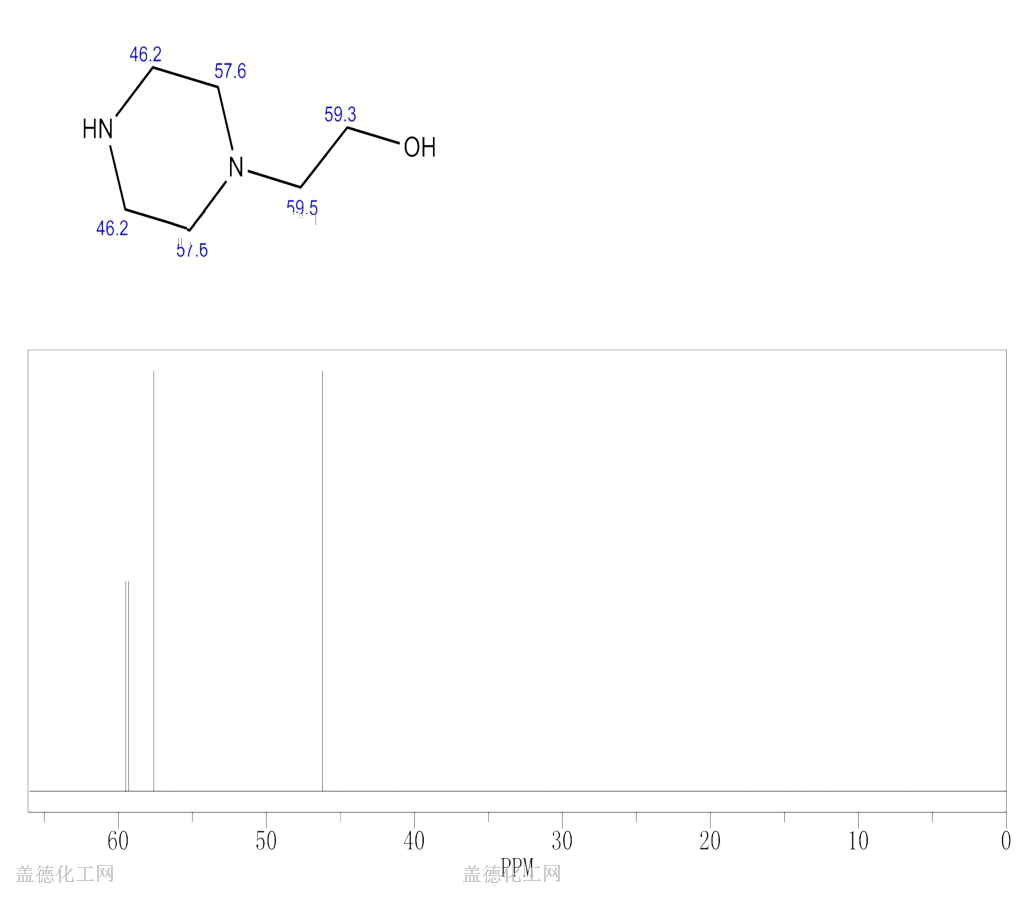
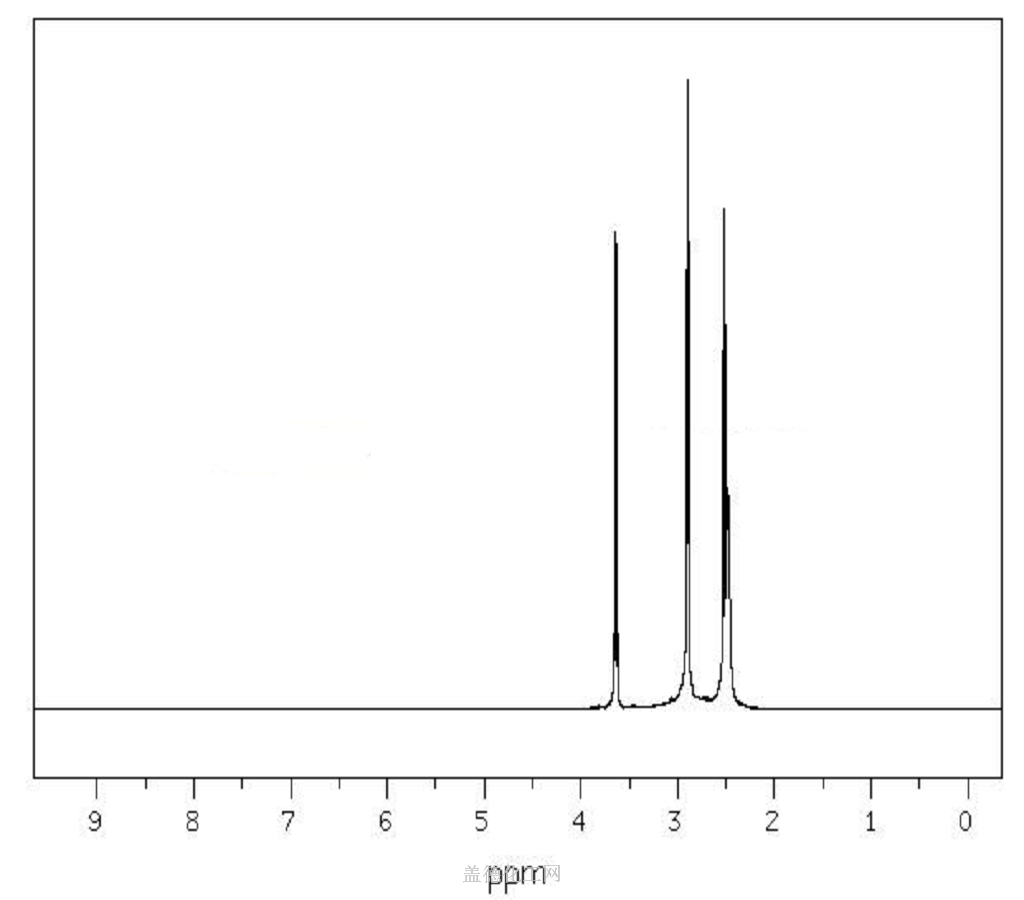
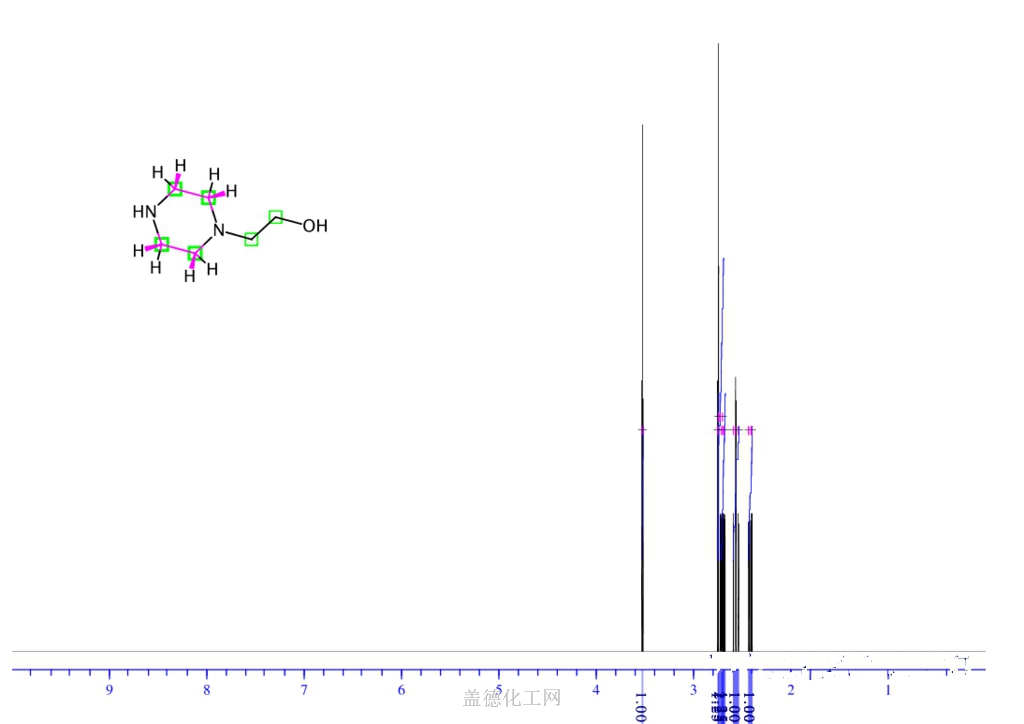
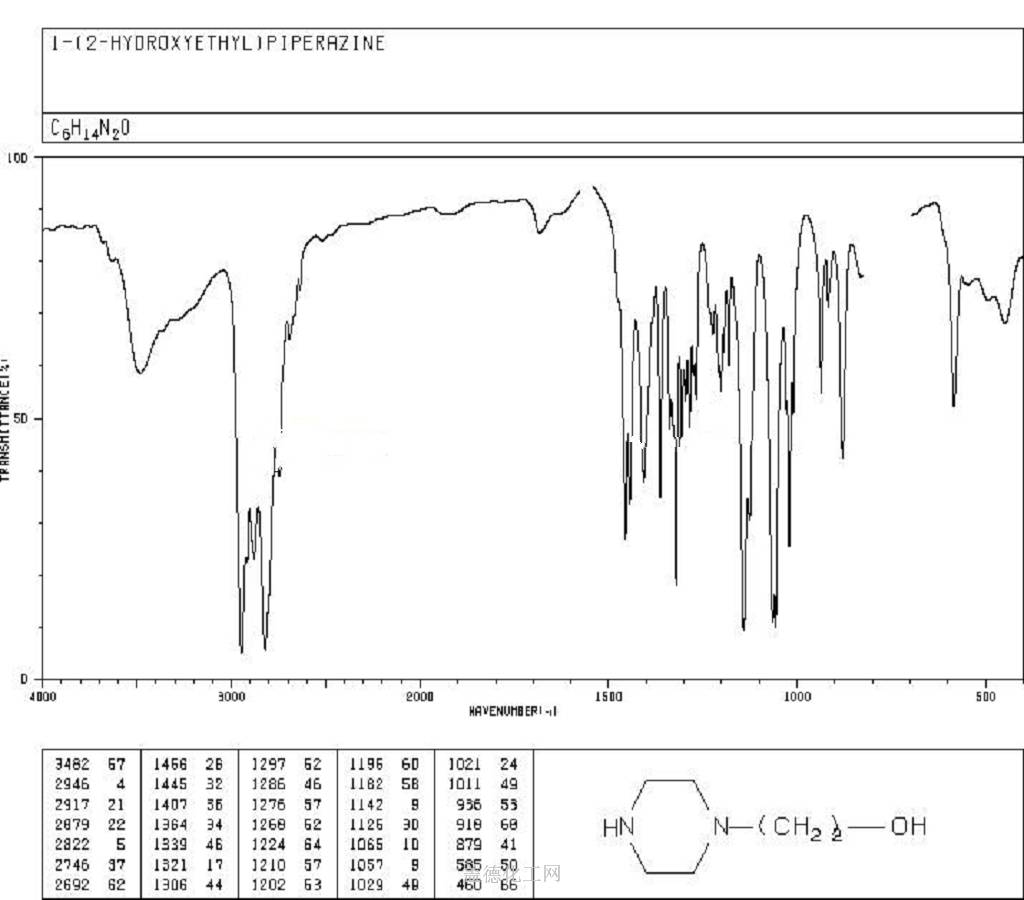
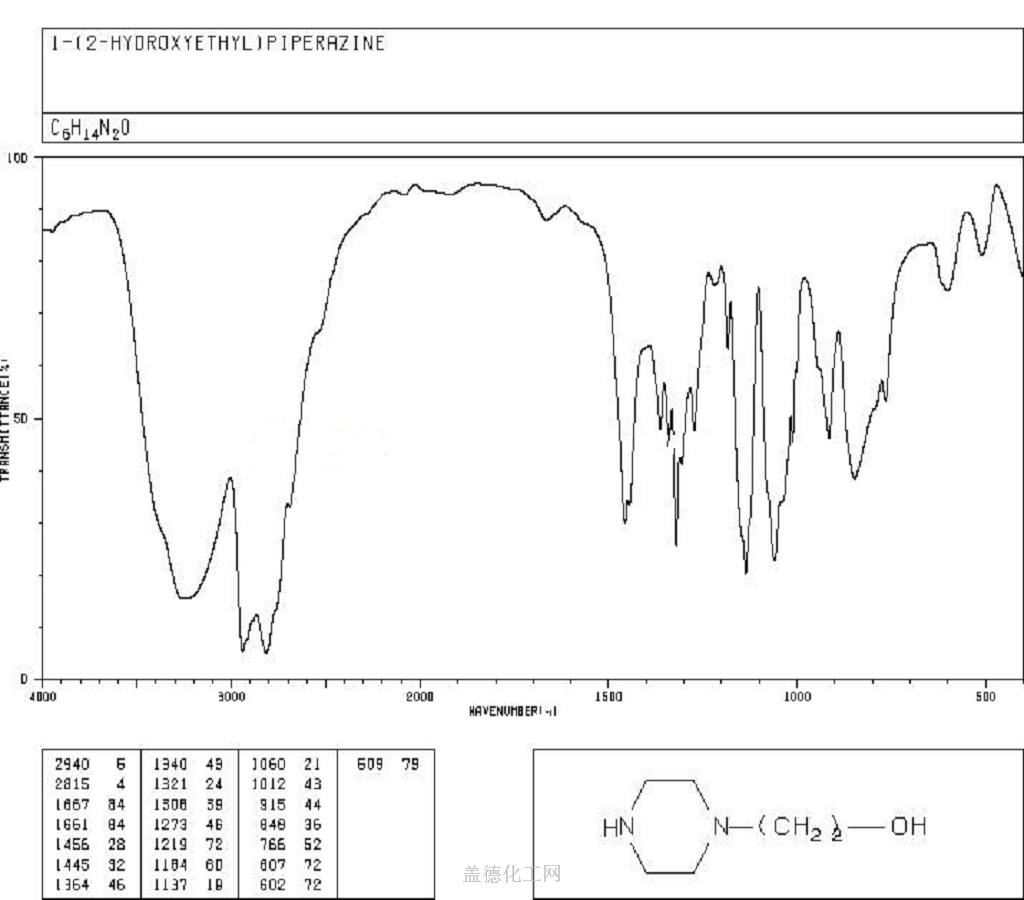
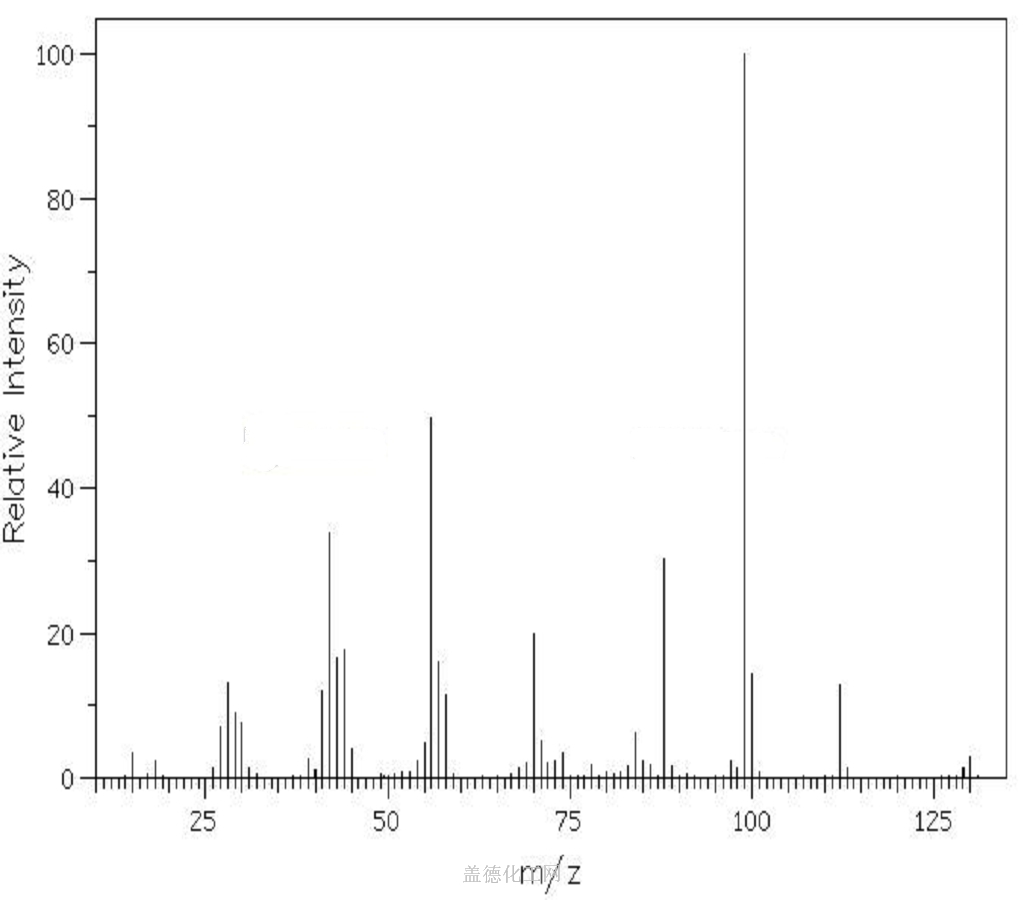
The 1-(2-Hydroxyethyl)piperazine, with its CAS registry number 103-76-4, has the IUPAC name of 2-piperazin-1-ylethanol. For being a kind of clear colorless to pale yellow oily liquid, it is usually applied in organic synthesis, with its product categories including Piperaizine.
The characteristics of this chemical are as below: (1)ACD/LogP: -0.45; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -3.43; (4)ACD/LogD (pH 7.4): -2.15; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 3; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 15.71; (13)Index of Refraction: 1.475; (14)Molar Refractivity: 36.22 cm3; (15)Molar Volume: 128.6 cm3; (16)Polarizability: 14.35×10-24 cm3; (17)Surface Tension: 34.7 dyne/cm; (18)Density: 1.012 g/cm3; (19)Flash Point: 101.9 °C; (20)Enthalpy of Vaporization: 56.01 kJ/mol; (21)Boiling Point: 245 °C at 760 mmHg; (22)Vapour Pressure: 0.00492 mmHg at 25°C; (23)Exact Mass: 130.110613; (24)MonoIsotopic Mass: 130.110613; (25)Topological Polar Surface Area: 35.5; (26)Heavy Atom Count: 9; (27)Complexity: 71.5; (28)Covalently-Bonded Unit Count: 1.
Production method of this chemical: 4-(2-hydroxy-ethyl)-piperazine-1-carboxylic acid 3-methyl-but-2-enyl ester could react to produce 2-piperazin-1-yl-ethanol. This?reaction could?happen in the presence of the reagent of diethylamine, the catalytic agent of Pd(0) (in situ from Pd(OAc)2 and m.sulfonated triphenylphosphine) and the solvent of H2O and acetonitrile. This?needs the?reaction time of 15 min in the condition of?5 molpercent catalyst.
Use of this chemical: 1-(2-Hydroxyethyl)piperazine could react with 2,3-dihydro-benzo[1,4]dioxine-2-carboxylic acid ethyl ester to produce (2,3-dihydro-benzo[1,4]dioxin-2-yl)-[4-(2-hydroxy-ethyl)-piperazin-1-yl]-methanone. This reaction needs the reaction time of 3 hour(s) and the reaction temp. of 110 ℃ with its?yield of?98%.
When you are dealing with this chemical, you should be cautious. For being a kind of irritant chemical which may cause inflammation to the skin or other mucous membranes, it is irritating to eyes, respiratory system and skin and may have risk of serious damage to eyes. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. And if in case of contact with eyes, rinse immediately with plenty of water and seek medical advice.?
Additionally, you could obtain the molecular structure by converting the following datas:
(1)Canonical SMILES: C1CN(CCN1)CCO
(2)InChI: InChI=1S/C6H14N2O/c9-6-5-8-3-1-7-2-4-8/h7,9H,1-6H2
(3)InChIKey: WFCSWCVEJLETKA-UHFFFAOYSA-N?
Below are the toxicity information of this chemical:
| Organism |
Test Type |
Route |
Reported Dose (Normalized Dose) |
Effect |
Source |
| guinea pig |
LD50 |
oral |
3720mg/kg (3720mg/kg) |
BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)
BEHAVIORAL: MUSCLE WEAKNESS
BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) |
Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 45(5), Pg. 67, 1980. |
| mouse |
LD50 |
intraperitoneal |
100mg/kg (100mg/kg) |
? |
National Technical Information Service. Vol. AD277-689, |
| rabbit |
LD50 |
oral |
3350mg/kg (3350mg/kg) |
BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)
BEHAVIORAL: MUSCLE WEAKNESS
BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) |
Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 45(5), Pg. 67, 1980. |
| rabbit |
LD50 |
skin |
> 5mL/kg (5mL/kg) |
? |
Union Carbide Data Sheet. Vol. 1/6/1970, |
| rat |
LD50 |
oral |
4920uL/kg (4.92mL/kg) |
? |
American Industrial Hygiene Association Journal. Vol. 23, Pg. 95, 1962.
? |

 EN
EN