Stable, but sensitive to moisture. Incompatible with strong acids, strong oxidizing agents, moisture.- 2.8 StorageTemp
- -20°C
3. Use and Manufacturing
- 3.1 Description
-
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, with the chemical formula C8H17N3·HCl, has the CAS number 25952-53-8. It is a white to off-white crystalline powder with a faint odor. The basic structure of this chemical consists of a carbodiimide group attached to a 3-dimethylaminopropyl group and an ethyl group. It is sparingly soluble in water. Safety information indicates that this compound may cause skin and eye irritation. It is also harmful if swallowed or inhaled. Precautions should be taken to avoid contact with skin, eyes, and clothing. In case of ingestion, immediate medical attention is required. This chemical should be handled in a well-ventilated area and with appropriate protective equipment.
Applicable Fields
Bioconjugation: 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is commonly used in bioconjugation reactions. Its purpose in this field involves the activation of carboxyl groups in biomolecules, allowing them to react with amino groups. The mechanism of action in bioconjugation is the formation of amide bonds between the activated carboxyl groups and the amino groups, resulting in the covalent attachment of biomolecules.
Peptide synthesis: This compound is widely used in peptide synthesis. Its purpose in this field is to activate carboxyl groups in amino acids, facilitating their coupling with other amino acids to form peptide bonds. The mechanism of action in peptide synthesis involves the formation of amide bonds between the activated carboxyl groups and the amino groups of the incoming amino acids, leading to the stepwise elongation of the peptide chain.
Storage
Conditions: Store in a cool and dry place.
- 3.2 GHS Classification
- Signal: Danger
GHS Hazard StatementsAggregated GHS information provided by 195 companies from 23 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 72 of 195 companies. For more detailed information, please visit ECHA C&L websiteOf the 22 notification(s) provided by 123 of 195 companies with hazard statement code(s): H302 (37.4%): Harmful if swallowed [ Warning Acute toxicity, oral] H315 (99.19%): Causes skin irritation [ Warning Skin corrosion/irritation] H317 (14.63%): May cause an allergic skin reaction [ Warning Sensitization, Skin] H318 (18.7%): Causes serious eye damage [ Danger Serious eye damage/eye irritation] H319 (80.49%): Causes serious eye irritation [ Warning Serious eye damage/eye irritation] H335 (95.93%): May cause respiratory irritation [ Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Precautionary Statement Codes
P261, P264, P270, P271, P272, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P310, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362, P363, P403+P233, P405, and P501
- 3.3 Usage
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is being used for the synthesis of amides. EDC.HCl is also used as a coupling agent in the preparation of esters from carboxylic acids using dimethylaminopyridine as the catalyst. It is water-soluble carbodiimide, widely used for peptide coupling.
4. Safety and Handling
- 4.1 Sensitive
- Hygroscopic
- 4.2 Toxicity
- LD50 intravenous in mouse: 56mg/kg
5. MSDS
2.Hazard identification 2.1 Classification of the substance or mixture Skin irritation, Category 2 Skin sensitization, Category 1 Eye irritation, Category 2 Specific target organ toxicity \u2013 single exposure, Category 3 2.2 GHS label elements, including precautionary statements | Pictogram(s) |  | | Signal word | Warning | | Hazard statement(s) | H315 Causes skin irritation H317 May cause an allergic skin reaction H319 Causes serious eye irritation H335 May cause respiratory irritation | | Precautionary statement(s) | | | Prevention | P264 Wash ... thoroughly after handling. P280 Wear protective gloves/protective clothing/eye protection/face protection. P261 Avoid breathing dust/fume/gas/mist/vapours/spray. P272 Contaminated work clothing should not be allowed out of the workplace. P271 Use only outdoors or in a well-ventilated area. | | Response | P302+P352 IF ON SKIN: Wash with plenty of water/... P321 Specific treatment (see ... on this label). P332+P313 If skin irritation occurs: Get medical advice/attention. P362+P364 Take off contaminated clothing and wash it before reuse. P333+P313 If skin irritation or rash occurs: Get medical advice/attention. P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P337+P313 If eye irritation persists: Get medical advice/attention. P304+P340 IF INHALED: Remove person to fresh air and keep comfortable for breathing. P312 Call a POISON CENTER/doctor/\u2026if you feel unwell. | | Storage | P403+P233 Store in a well-ventilated place. Keep container tightly closed. P405 Store locked up. | | Disposal | P501 Dispose of contents/container to ... | 2.3 Other hazards which do not result in classification none
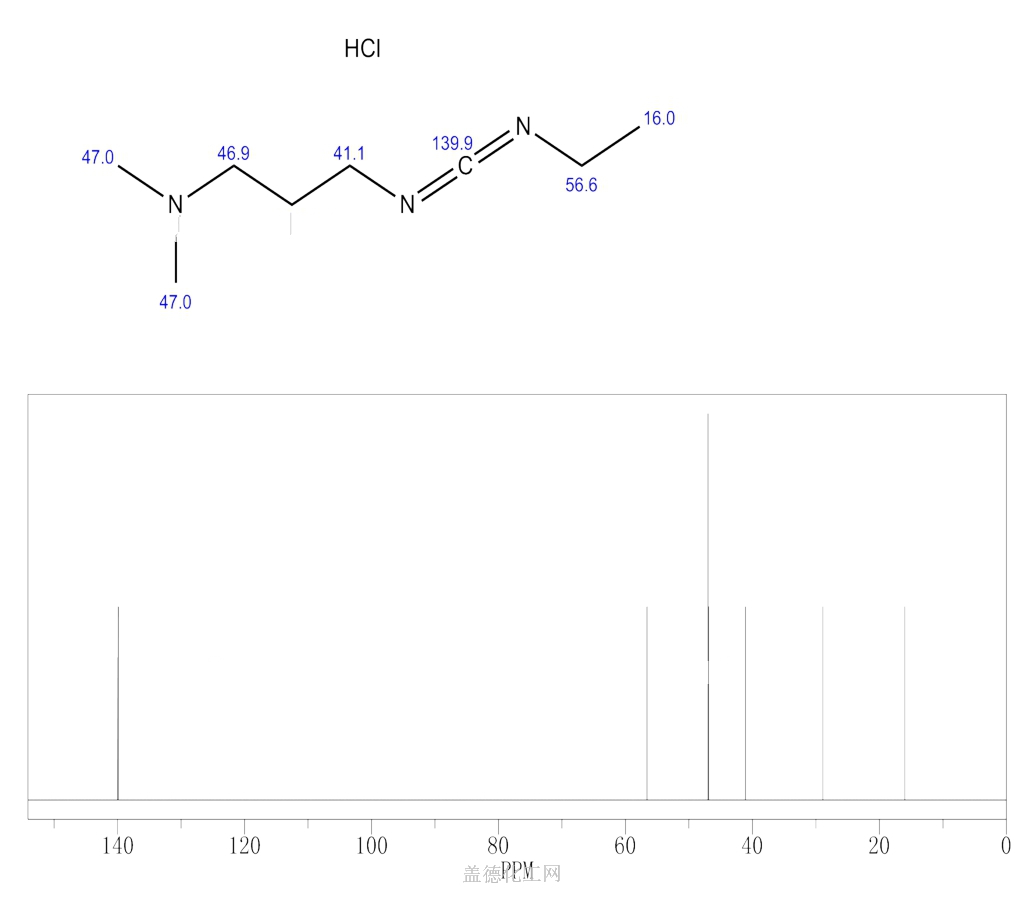
6. NMR Spectrum
7. Computed Properties
- Molecular Weight:191.703g/mol
- Molecular Formula:C8H18ClN3
- Compound Is Canonicalized:False
- XLogP3-AA:191.1189253
- Monoisotopic Mass:191.1189253
- Complexity:134
- Rotatable Bond Count:5
- Hydrogen Bond Donor Count:1
- Hydrogen Bond Acceptor Count:3
- Topological Polar Surface Area:28
- Heavy Atom Count::12
- Defined Atom Stereocenter Count:0
- Undefined Atom Stereocenter Count:0
- Defined Bond Stereocenter Count:0
- Undefined Bond Stereocenter Count:0
- Isotope Atom Count:0
- Covalently-Bonded Unit Count:2
- CACTVS Substructure Key Fingerprint:AAADceBzAAAEAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAHAAAAAAACADBAAQDAAMAAAAgAAAAJAAAAAAAAAAAAAAIAAAAAAAAQAAEAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA==
8.Other Information
- Usage
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is used as a carboxyl activating agent. It is used in peptide synthesis, 3'-amino-3'-deoxyadenosine-5'-di- and triphosphates and in the preparation of antibodies like immunoconjugates. It plays a vital role for immobilization of large biomolecules in association with N-hydroxysuccinimide. It is also used in the acylation of phosphoranes.
- BRN
- 5764110
- Chemical Properties
- 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC-HCl) is a white crystalline powder, easily deliquescent, soluble in water >20g/100ml, soluble in ethanol, used as an activating reagent for carboxyl groups in amide synthesis, also used for activating phosphate groups, cross-linking of proteins and nucleic acids, and the production of immunocouplers. EDC-HCl is used in the pH range of 4.0-6.0 and is often used in conjunction with N-Hydroxysuccinimide (NHS) or N-Hydroxysulfosuccinimide sodium salt to improve coupling efficiency.
- Uses
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is being used for the synthesis of amides. EDC.HCl is also used as a coupling agent in the preparation of esters from carboxylic acids using dimethylaminopyridine as the catalyst. It is water-soluble carbodiimide, widely used for peptide coupling.
- General Description
-
Water-soluble derivative of carbodiimide useful for conjugating haptens to proteins and polypeptides. Used to modify NMDA receptors and as a condensing agent in peptide synthesis. The major advantage of EDAC coupling is the easy removal of excess reagent and the corresponding urea by washing with dilute acid or water. Carbodiimides catalyze the formation of amide bonds, carboxylic acids, and amines by activating the carboxylate to form an O-acylurea. This intermediate can be attacked by an amine directly to form an amide. EDAC is released as a soluble urea derivative.
-
Tel:
Update Time:2024/11/12
-
Tel:
Update Time:2024/11/15
-
Tel:
Update Time:2024/11/15
-
Tel:
Update Time:2024/11/15
-
Tel:
Update Time:2024/11/15
10. Related Questions
- What are the uses and synthesis method of 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride? Description Carbodiimide reagents, such as 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl), N, N'-diisopropyl carbodiimide (DIC) and N, N'-dicyclohexylcarbodiimide (DCC), accele..
- What is 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride?1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, also known as EDC hydrochloride, is a white crystalline powder that easily absorbs moisture. It has a solubility of >20g/100ml in water and..
- What is the purification method for 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride?1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is a condensing agent and cross-linking agent belonging to the carbodiimide series. It is mainly used in organic synthesis, such as the synt..
- What is 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and its applications?1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, also known as EDC hydrochloride, has the molecular formula C8H17N3·HCl and a relative molecular mass of 191.70. It is a white crystalline p..
11. Realated Product Infomation
1. Names and Identifiers
2. Properties
| 
 EN
EN