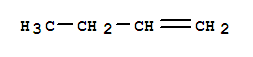1-BUTENE, with the chemical formula C4H8 and CAS registry number 106-98-9, is a compound known for its applications in various industries. This colorless gas, also referred to as alpha-Butylene, is characterized by its double bond between the first and second carbon atoms. It is commonly used as a starting material in the production of polymers, such as polyethylene and polypropylene. 1-BUTENE is also used as a fuel and as a solvent in various chemical processes. It is important to handle this compound with caution, as it is flammable and can cause irritation to the respiratory system. Overall, 1-BUTENE plays a crucial role in the manufacturing of various products and serves as a building block for many chemical reactions.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. Safety and Handling
5. MSDS
6. NMR Spectrum
7. Synthesis Route
8. Precursor and Product
9. Computed Properties
12. Related Questions
13. Realated Product Infomation

 EN
EN