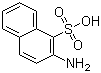
-

2-Aminonaphthalene-1-sulfonic acid
- CAS:81-16-3
- MW:223.24836
- MF:C10H9NO3S
- Category
Organic Intermediates Pharmaceuticals and Biochemicals Basic Organic Chemicals Agrochemicals Inorganic Chemicals Catalyst and Auxiliary Custom Manufacturing Food & Feed Additives Daily Chemicals Dyestuffs and Pigments Laboratory Chemicals Adhesives and Sealants Flavour & Fragrance Paint and Coatings Polymer Metals and Minerals
- Encyclopedia
- Dictionary
- Supplier
- Buyer Request
- TradeShow
- FAQs
- Structure Search
 EN
EN