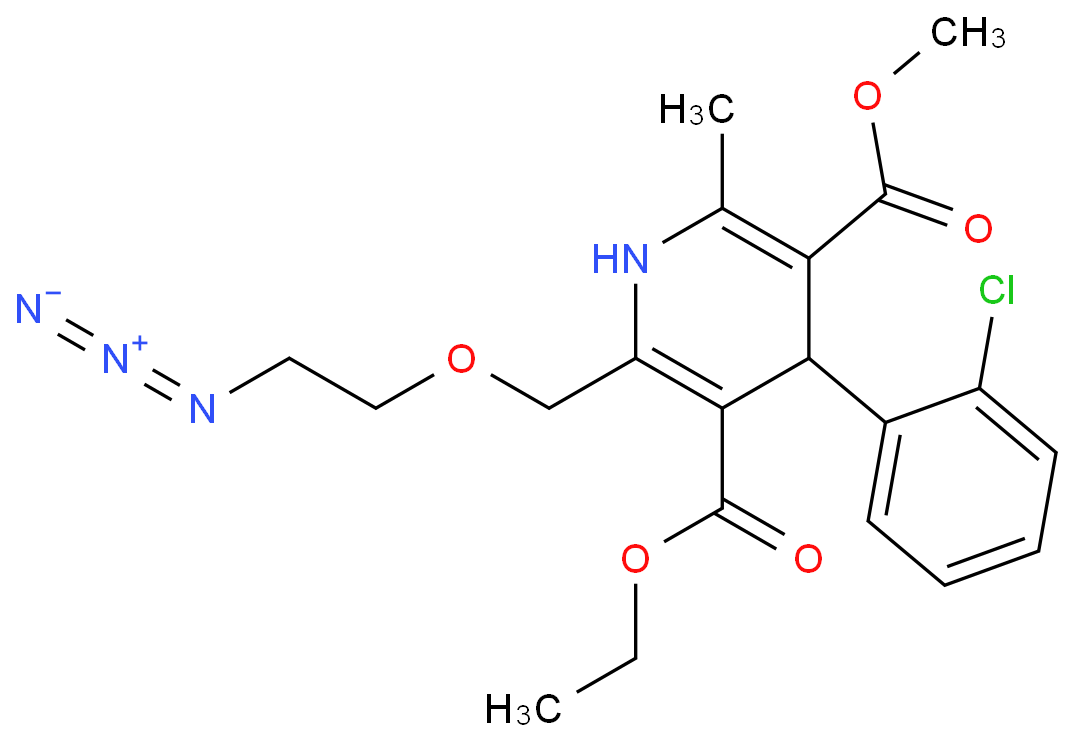
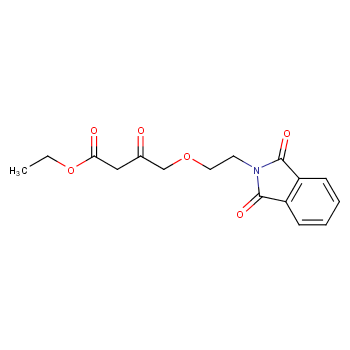
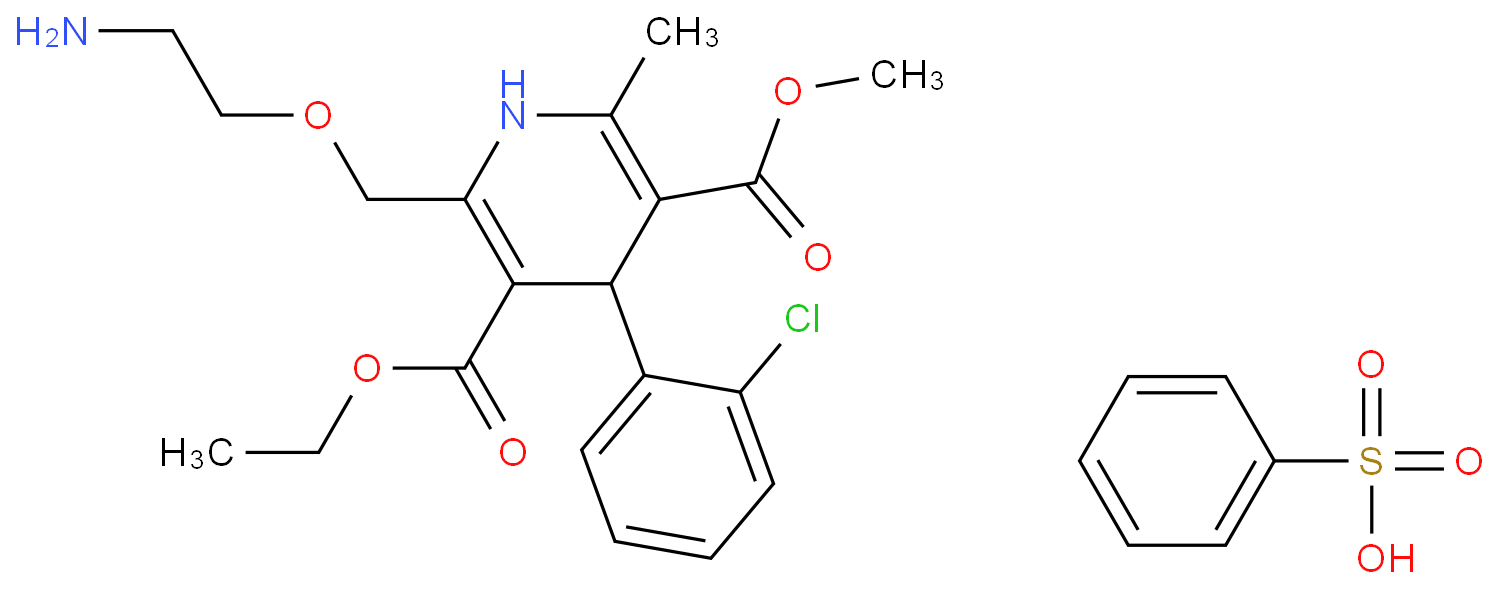
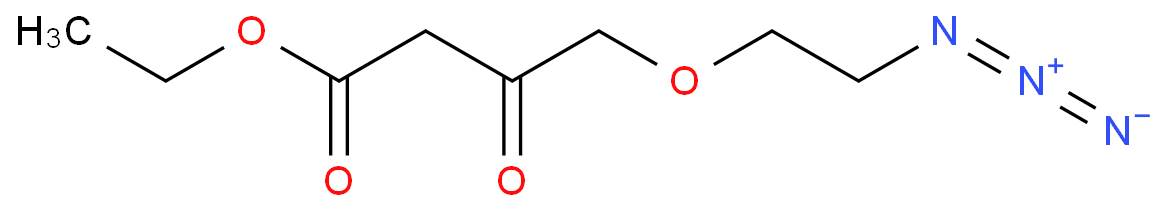
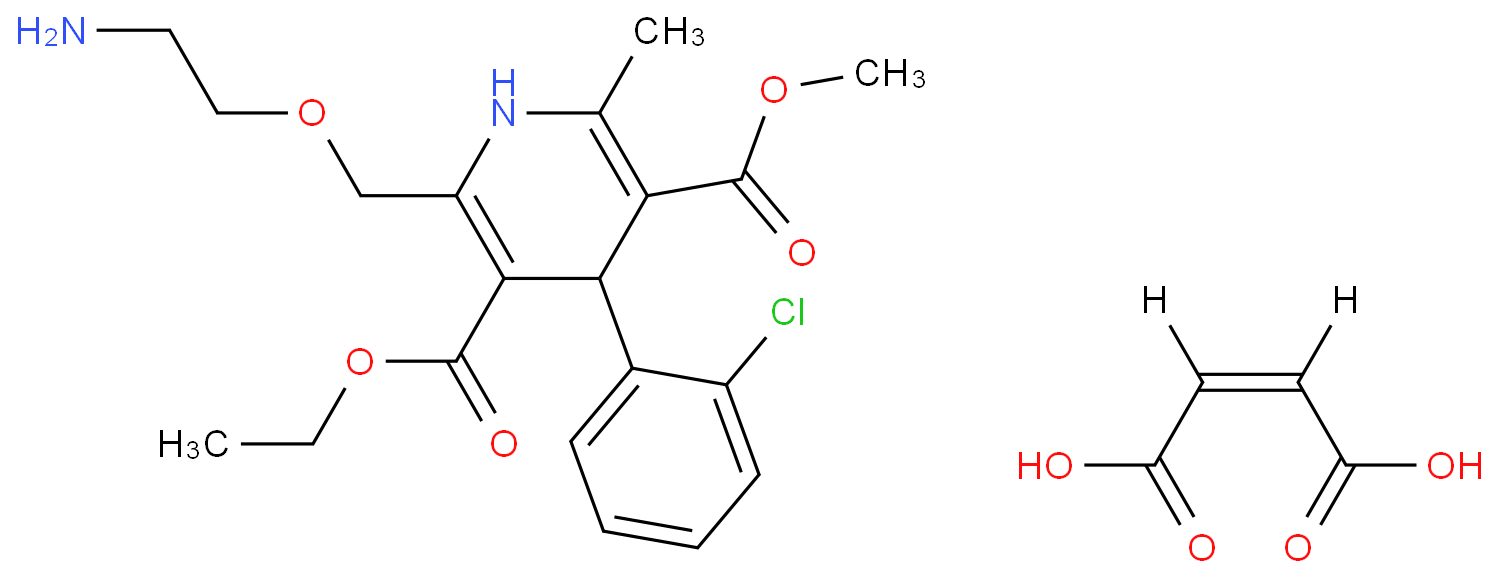
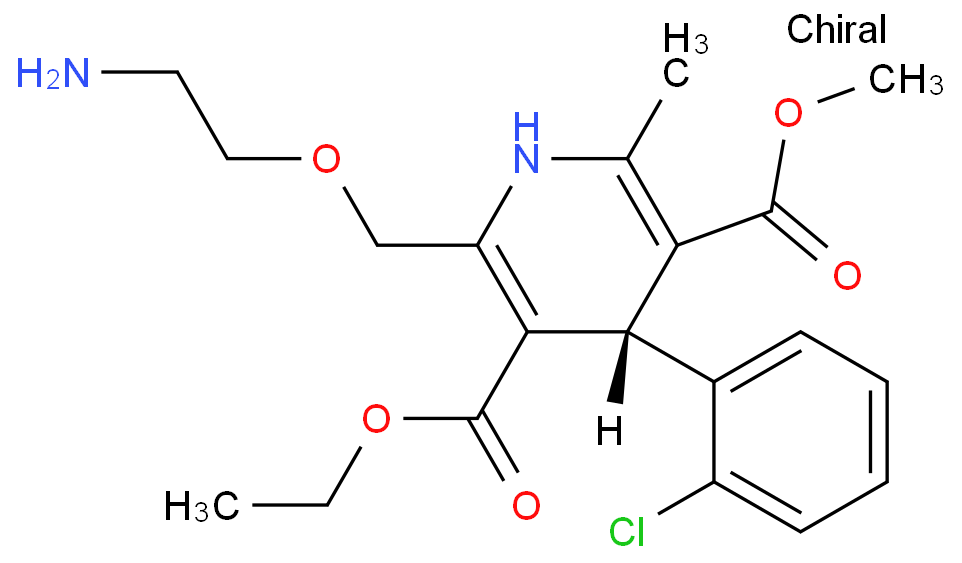
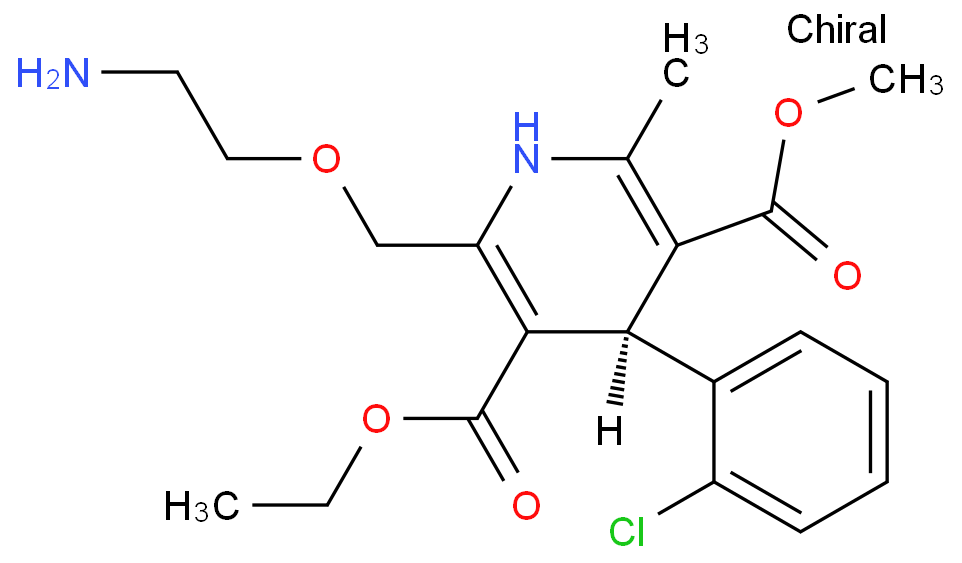
The Amlodipine with the cas number 88150-42-9, is also called (1)3-Ethyl-5-methyl ( -)-2-((2-aminoethoxy)methyl)-4-(2-chlorphenyl)-1,4-dihydro-6-methyl-3,5-pyridindicarboxylat ; (2)3-Ethyl-5-methyl ( -)-2-((2-aminoethoxymethyl)-4-(o-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate ; (3) Amlocard ; (4) Amlodipine ; (5) Amlodipino ; (6) Amlodipinum ; (7) Amlodis ; (8) Amlor ; (9) Coroval ; (10) Lipinox ; (11) Norvasc. It belongs to the following product categories: (1)Active Pharmaceutical Ingredients; (2)Dihydropyridine Class Chemicals; (3)Intermediates & Fine Chemicals; (4)Pharmaceuticals; (5)Calcium channel
Properties of Amlodipine are: (1)ACD/LogP: 4.16 ; (2)# of Rule of 5 Violations: 0 ; (3)ACD/LogD (pH 5.5): 1.23 ; (4)ACD/LogD (pH 7.4): 2.64 ; (5)ACD/BCF (pH 5.5): 1 ; (6)ACD/BCF (pH 7.4): 26.11 ; (7)ACD/KOC (pH 5.5): 5.14 ; (8)ACD/KOC (pH 7.4): 133.65 ; (9)#H bond acceptors: 7 ; (10)#H bond donors: 3 ; (11)#Freely Rotating Bonds: 11 ; (12)Polar Surface Area: 68.31??2 ; (13)Index of Refraction: 1.545 ; (14)Molar Refractivity: 105.41 cm3 ; (15)Molar Volume: 333 cm3 ; (16)Polarizability: 41.79 ×10-24cm3 ; (17)Surface Tension: 44.4 dyne/cm ; (18)Density: 1.227 g/cm3 ; (19)Flash Point: 272.6 °C ; (20)Enthalpy of Vaporization: 80.17 kJ/mol ; (21)Boiling Point: 527.2 °C at 760 mmHg ; (22)Vapour Pressure: 3.34E-11 mmHg at 25°C
The Amlodipine appears to be Yellow Solid. It is used as an anti-hypertensive and in the treatment of angina because it is a long-acting calcium channel blocker. Seemimg Like other calcium channel blockers, Amlodipine reduce blood pressure by relaxing the smooth muscle in the arterial wall, decreasing peripheral resistance. Besides, it increases blood flow to the heart muscle in angina. Adverse effcts include common headache, fatigue, insomnia, edema, abdominal pain, nausea, dizziness, palpitation and red face. Rare rash, itching, expiratory dyspnea, muscle spasm and indigestion. Rarely have myocardial infarction and chest. It can have edema, headache, dizziness, weakness, etc.
The Amlodipine can react with nicotinic acid to obtain 4-(2-chloro-phenyl)-2-methyl-6-{2-[(pyridine-3-carbonyl)-amino]-ethoxymethyl}-1,4-dihydro-pyridine-3,5-dicarboxylic acid 5-ethyl ester 3-methyl ester.
Reaction condition is CH2Cl2 as solvent under ambient temperature for 16 hour(s). Yield is 57 %.
?????????????????
?
You can still convert the following datas into molecular structure :
1. SMILES: Clc1ccccc1C2C(=C(/N/C(=C2/C(=O)OCC)COCCN)C)\C(=O)OCCopyCopied
2. InChI:?InChI=1/C20H25ClN2O5/c1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21/h5-8,17,23H,4,9-11,22H2,1-3H3
The Amlodipine toxic data can be showed in the following sheet.
?
| Organism |
Test Type |
Route |
Reported Dose (Normalized Dose) |
Effect |
Source |
| child |
TDLo |
oral |
400ug/kg (0.4mg/kg) |
VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION |
American Journal of Emergency Medicine. Vol. 18, Pg. 581, 2000. |
| women |
LDLo |
oral |
1400ug/kg (1.4mg/kg) |
CARDIAC: PULSE RATE INCREASE WITHOUT FALL IN BP
VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION |
Journal of Toxicology, Clinical Toxicology. Vol. 33, Pg. 253, 1995. |
| women |
TDLo |
oral |
600ug/kg/3D-I (0.6mg/kg) |
SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION
BLOOD: HEMORRHAGE
BLOOD: THROMBOCYTOPENIA |
Annals of Pharmacotherpy. Vol. 33, Pg. 1126, 1999. |
 EN
EN