1. GB2760-1996 stipulates it as allowable usable spices in food. It is mainly used for the preparation of vanilla, fennel and beer flavor. 2. It is used for analyzing the reagents, solvents, and used for preparing perfumery and enteral pesticides. 3. It is used for the production of perfumes, dyes, pharmaceuticals, pesticides, also used as a solvent.4. It is used in organic synthesis, also used as solvents, perfume and insect repellent. 5. It is used as solvents for recrystallization, fillers of thermostat, and used for measuring refractive index, as spices and organic synthesis intermediates.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. Safety and Handling
5. MSDS
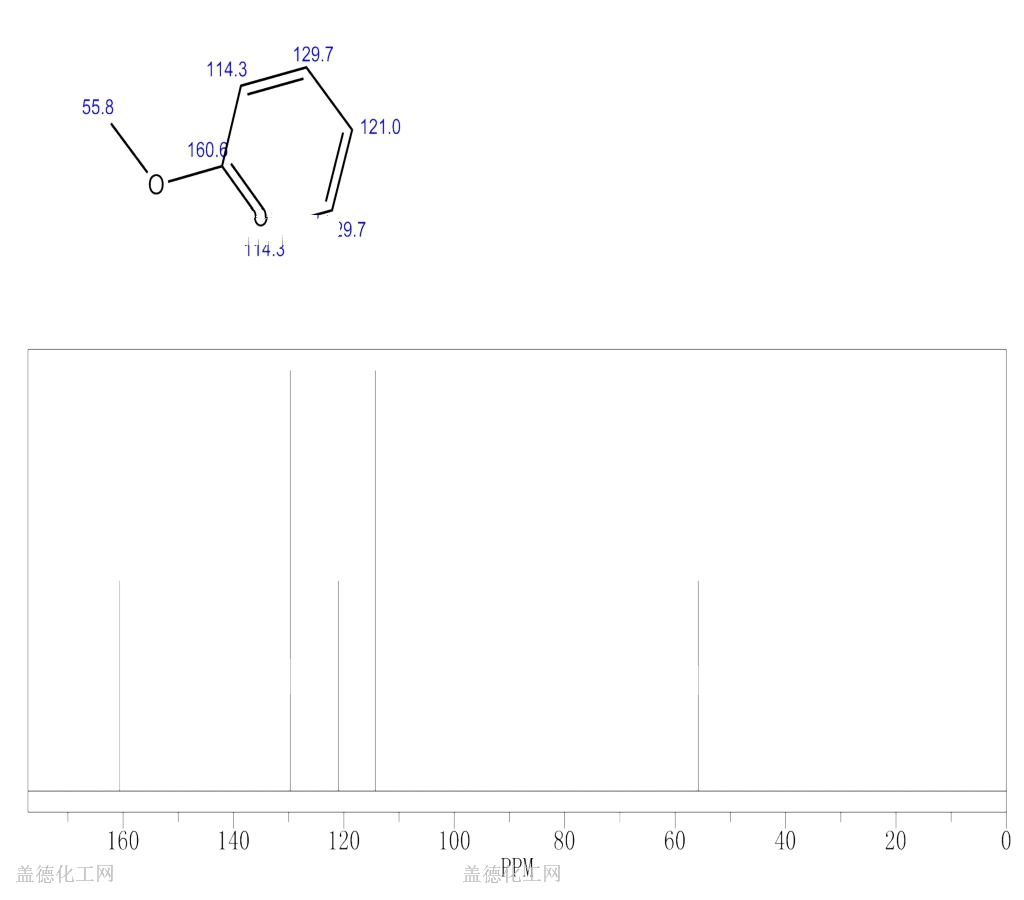
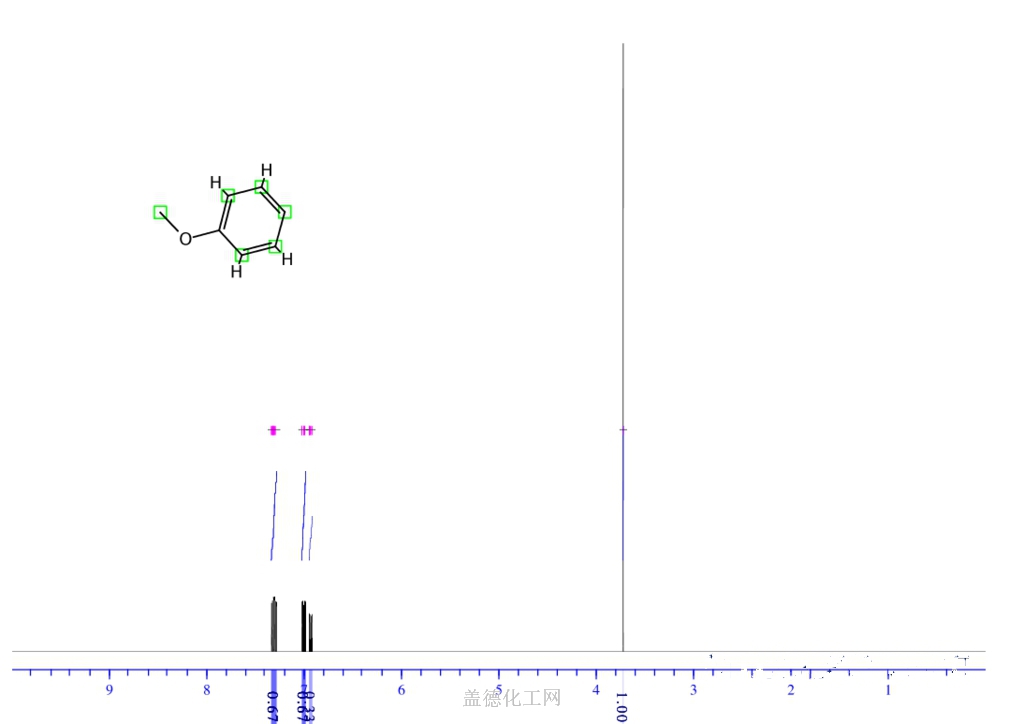
6. NMR Spectrum
7. Synthesis Route
8. Precursor and Product
9. Computed Properties
12. Related Questions
13. Realated Product Infomation

 EN
EN