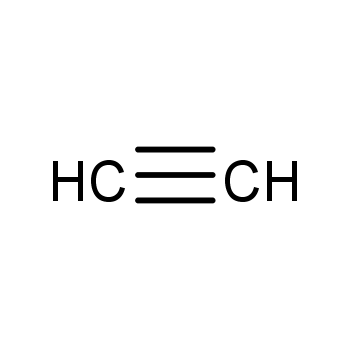
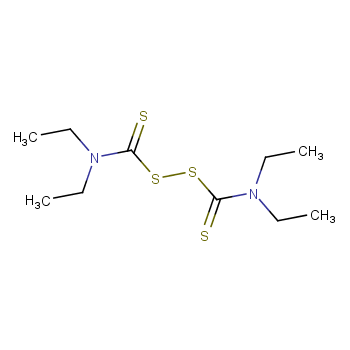
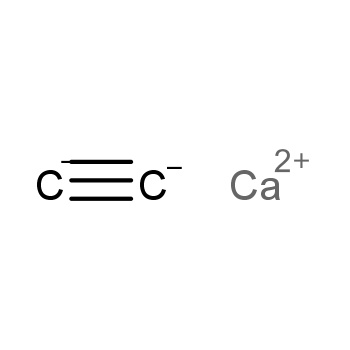
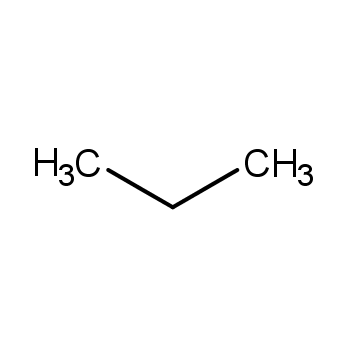
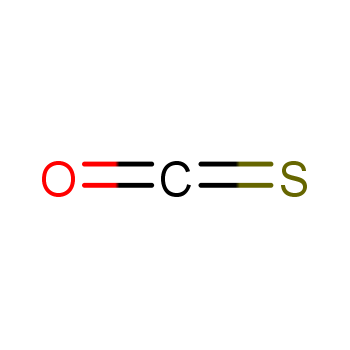
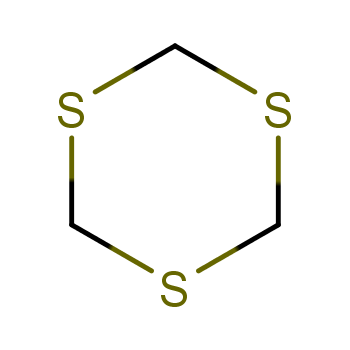
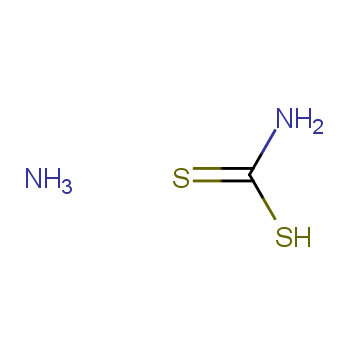
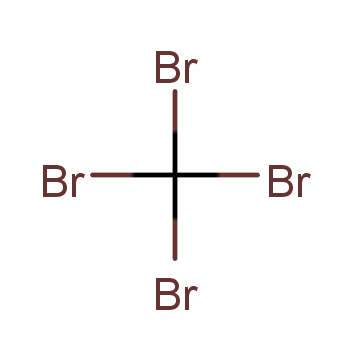
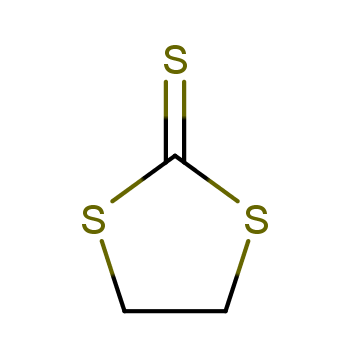
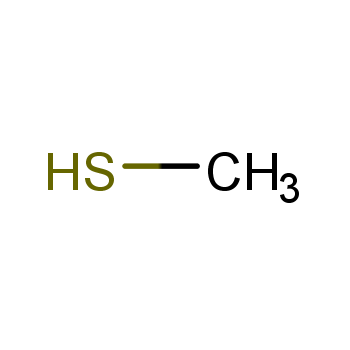
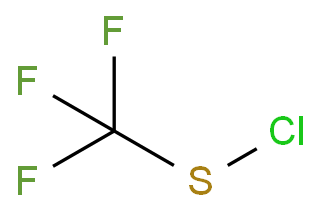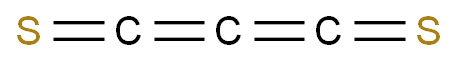| P210 Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P233 Keep container tightly closed. P240 Ground and bond container and receiving equipment. P241 Use explosion-proof [electrical/ventilating/lighting/...] equipment. P242 Use non-sparking tools. P243 Take action to prevent static discharges. P280 Wear protective gloves/protective clothing/eye protection/face protection. P264 Wash ... thoroughly after handling. P260 Do not breathe dust/fume/gas/mist/vapours/spray. P270 Do not eat, drink or smoke when using this product. P201 Obtain special instructions before use. P202 Do not handle until all safety precautions have been read and understood. | 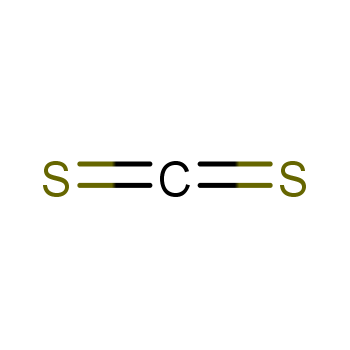
 EN
EN