The Cobalt, with the cas registry number 7440-48-4, is a kind of steel grey chemical. This chemical is insoluble in water, and it is stable chemically but incompatible with acetylene, hydrazinium nitrate, oxidizing agents, acids. Its product categories are various, including Metals; Inorganics; Catalysis and Inorganic Chemistry; Chemical Synthesis; CobaltMetal and Ceramic Science; Cobalt; Metal and Ceramic Science; C-D, Puriss p.a.Chemical Synthesis; Analytical Reagents for General Use; Puriss p.a.
The physical properties of this chemical are below: (1)#H bond acceptors: 0; (2)#H bond donors: 0; (3)#Freely Rotating Bonds: 0; (4)Polar Surface Area: 0; (5)Exact Mass: 58.9332; (6)MonoIsotopic Mass: 58.9332; (7)Heavy Atom Count: 1; (8)Covalently-Bonded Unit Count: 1.
As to its usage, it is widely applied in many ways. This chemical is usually used in the preparation of magnetic, wear-resistant, and high-strength alloys; It is also used in paints, varnishes, and inks as drying agents through the oxidation of certain compounds.
When you are using this chemical, you should be very cautious. For one thing, it is harmful which may cause damage to health. This is irritating to eyes and respiratory system and skin and may cause sensitization by inhalation and skin contact. For another thing, it is toxic which may at low levels cause damage to health. If by inhalation, in contact with skin and if swallowed, it will be very dangerous to our body. Besides, it may cause cancer and has limited evidence of a carcinogenic effect. Besides all these, it is highly flammable which may catch fire in contact with air, only needing brief contact with an ignition source, and it has a very low flash point or evolve highly flammable gases in contact with water. What's more, it may cause long-term adverse effects in the aquatic environment.
Due to so many dangers, you could take the different measures to deal with different cases. Wear suitable protective clothing, gloves and eye/face protection. If in case of contact with eyes, rinse immediately with plenty of water and seek medical advice, and if in case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) Then do not not breathe vapour and dust and remember to avoid exposure - obtain special instructions before use. Besides, avoid release to the environment and you could also refer to special instructions / safety data sheets. When store this chemical, keep contents under ... (there follows the name of a liquid).?
Additionally, you could convert the following datas into the molecular structure:
(1)Canonical SMILES: [Co]
(2)InChI: InChI=1S/Co
(3)InChIKey: GUTLYIVDDKVIGB-UHFFFAOYSA-N??
Below are the toxicity information of this chemical:
| Organism |
Test Type |
Route |
Reported Dose (Normalized Dose) |
Effect |
Source |
| mouse |
LDLo |
intraperitoneal |
100mg/kg (100mg/kg) |
? |
Environmental Quality and Safety, Supplement. Vol. 1, Pg. 1, 1975.
? |
| rabbit |
LDLo |
intravenous |
100mg/kg (100mg/kg) |
? |
Environmental Quality and Safety, Supplement. Vol. 1, Pg. 1, 1975.
? |
| rabbit |
LDLo |
oral |
750mg/kg (750mg/kg) |
BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) |
Archives Internationales de Pharmacodynamie et de Therapie. Vol. 62, Pg. 347, 1939. |
| rat |
LD50 |
intraperitoneal |
100mg/kg (100mg/kg) |
VASCULAR: REGIONAL OR GENERAL ARTERIOLAR OR VENOUS DILATION
LIVER: OTHER CHANGES
BLOOD: OTHER CHANGES |
Industrial Medicine. Vol. 15, Pg. 482, 1946. |
| rat |
LD50 |
oral |
6171mg/kg (6171mg/kg) |
BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)
BEHAVIORAL: ATAXIA
GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" |
Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 1, Pg. 686, 1992. |
| rat |
LDLo |
intratracheal |
25mg/kg (25mg/kg) |
? |
National Technical Information Service. Vol. AEC-TR-6710, |
| rat |
LDLo |
intravenous |
100mg/kg (100mg/kg) |
? |
Environmental Quality and Safety, Supplement. Vol. 1, Pg. 1, 1975.
? |
?
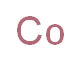
 EN
EN