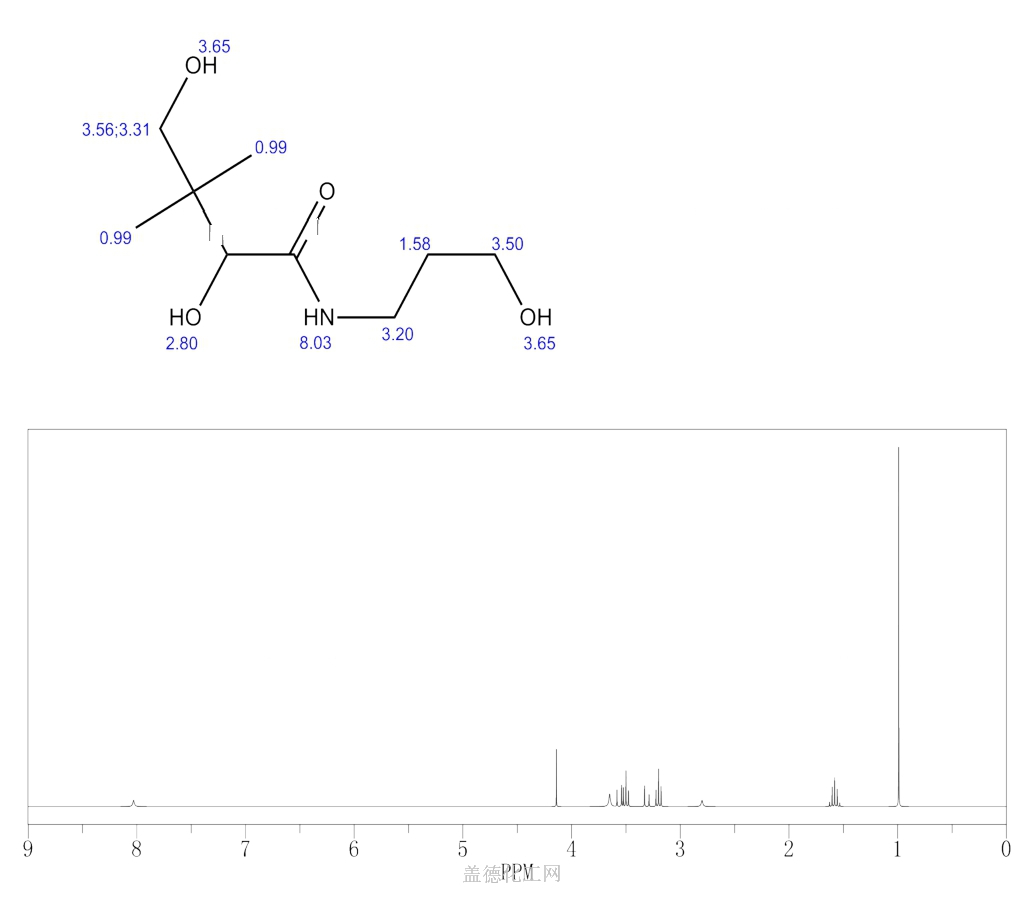
Dexpanthenol (CAS 81-13-0) is a clear, viscous liquid that is commonly used in various industries. Its chemical structure consists of a pantothenic acid derivative with an alcohol group, making it a stable and water-soluble compound. Dexpanthenol is highly soluble in water and has a slightly sweet taste. It is also known as provitamin B5, as it can be converted into vitamin B5 (pantothenic acid) in the body.
Applicable Fields
Pharmaceutical Industry: Dexpanthenol is widely used in the pharmaceutical industry for its therapeutic properties. It is commonly used in topical formulations such as creams, ointments, and lotions, as it has moisturizing and soothing effects on the skin. Dexpanthenol is also used in oral medications and supplements for its role in promoting skin health and wound healing.
Cosmetics Industry: In the cosmetics industry, dexpanthenol is a popular ingredient in skincare and haircare products. It is known for its moisturizing and hydrating properties, making it suitable for products such as moisturizers, serums, shampoos, and conditioners. Dexpanthenol helps to improve the skin's barrier function, reduce transepidermal water loss, and enhance the appearance and texture of hair.
Mechanism of Action
Dexpanthenol functions as a prodrug, meaning it is converted into its active form (pantothenic acid) in the body. Pantothenic acid is an essential nutrient that plays a crucial role in various biological processes, including the synthesis of coenzyme A (CoA). CoA is involved in numerous metabolic reactions, such as the production of energy from carbohydrates, fats, and proteins. Dexpanthenol's moisturizing and wound healing effects are attributed to its ability to enhance the skin's natural barrier function and promote tissue repair.
Storage Conditions
Store in a cool, dry place.

 EN
EN

 Xn
Xn