-
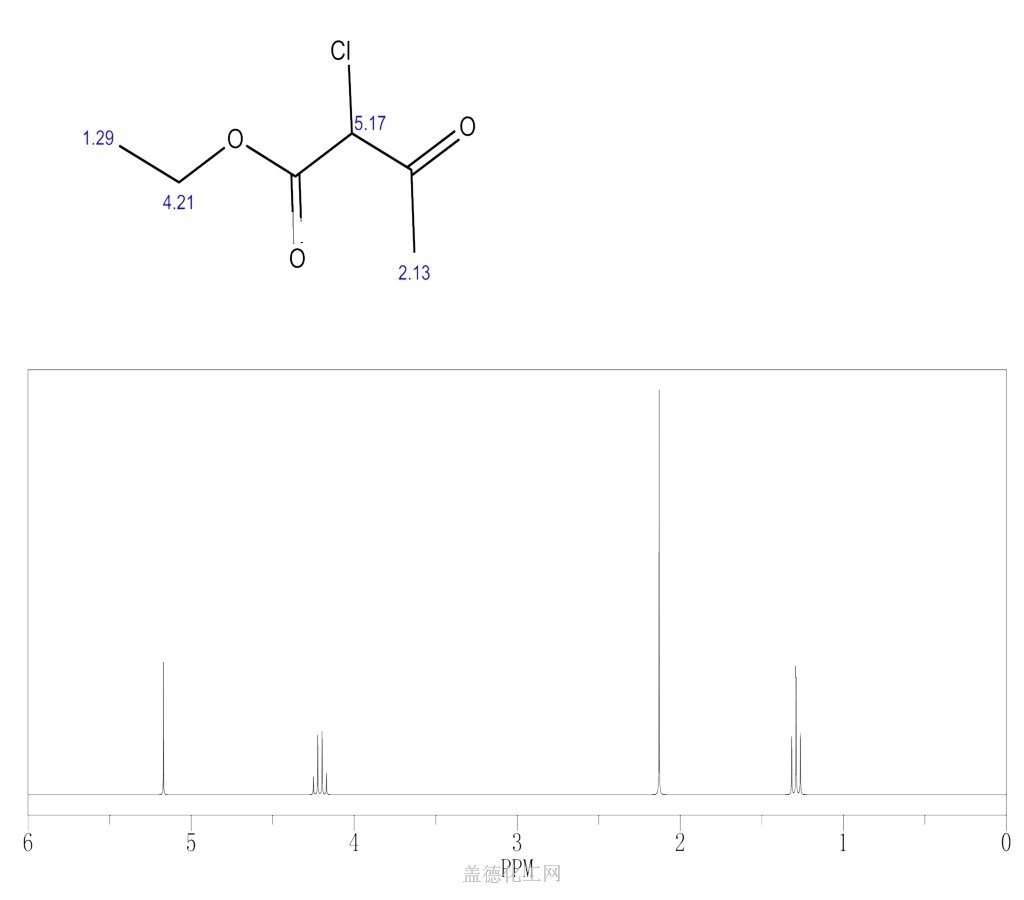
Ethyl 2-chloroacetoacetate
Ethyl 2-chloroacetoacetate, with the chemical formula C6H9ClO3 and CAS registry number 609-15-4, is a compound known for its applications in organic synthesis. This colorless liquid, also referred to as ethyl chloroacetoacetate, is characterized by its chlorine and acetoacetate functional groups. It is commonly used as a reagent in the preparation of various organic compounds, including pharmaceutical intermediates and agrochemicals. Ethyl 2-chloroacetoacetate is known for its ability to undergo condensation reactions, making it a valuable building block in the synthesis of complex molecules. It is important to handle this compound with caution, as it is flammable and may cause irritation to the skin, eyes, and respiratory system. Overall, ethyl 2-chloroacetoacetate plays a crucial role in the field of organic chemistry, offering a versatile platform for the introduction of chlorine and acetoacetate moieties into different molecules.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. MSDS
5. NMR Spectrum
6. Synthesis Route
7. Precursor and Product
8. Computed Properties
11. Related Questions
12. Realated Product Infomation

 EN
EN