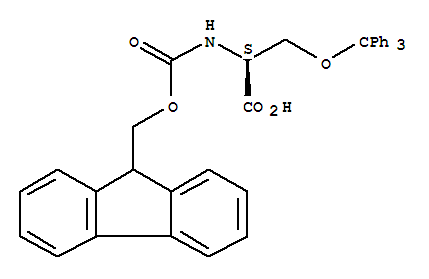
Fmoc-O-trityl-L-serine, with the chemical formula C51H45NO5 and CAS registry number 111061-56-4, is a compound known for its applications in peptide synthesis. This compound is a derivative of serine, an amino acid commonly found in proteins. Fmoc-O-trityl-L-serine is often used as a protecting group in peptide synthesis, providing stability to the amino acid during the synthesis process. It is characterized by the Fmoc (9-fluorenylmethoxycarbonyl) protecting group and the trityl (triphenylmethyl) protecting group. These groups can be selectively removed to expose the reactive amino group for further peptide chain elongation. Fmoc-O-trityl-L-serine is an important tool in the field of peptide chemistry, allowing for the efficient and controlled synthesis of complex peptides and proteins.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. MSDS
5. Synthesis Route

 EN
EN