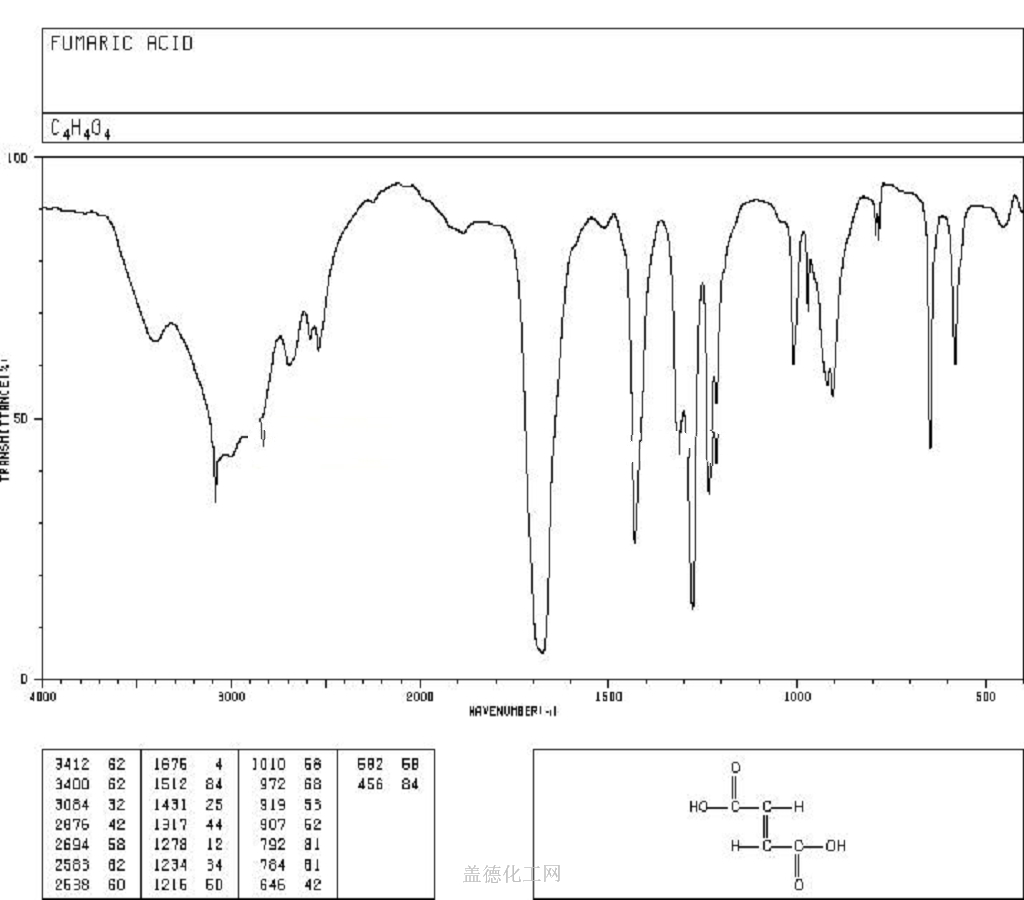
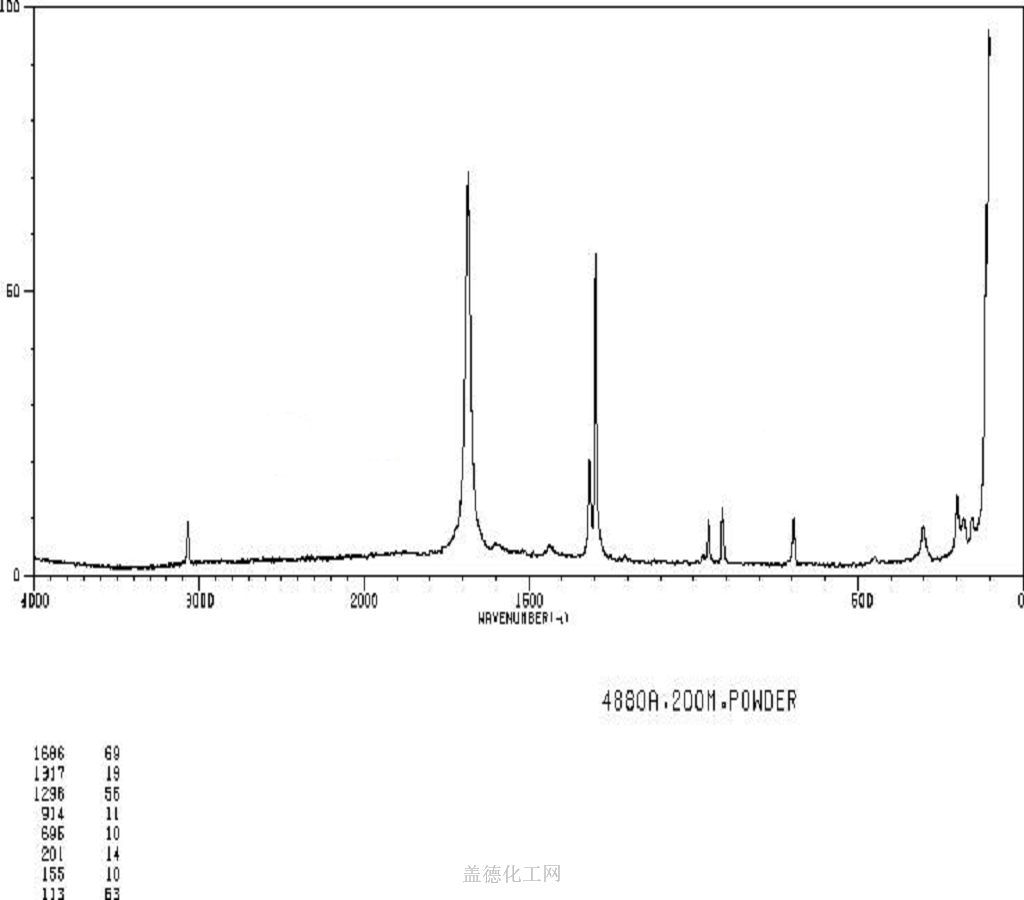
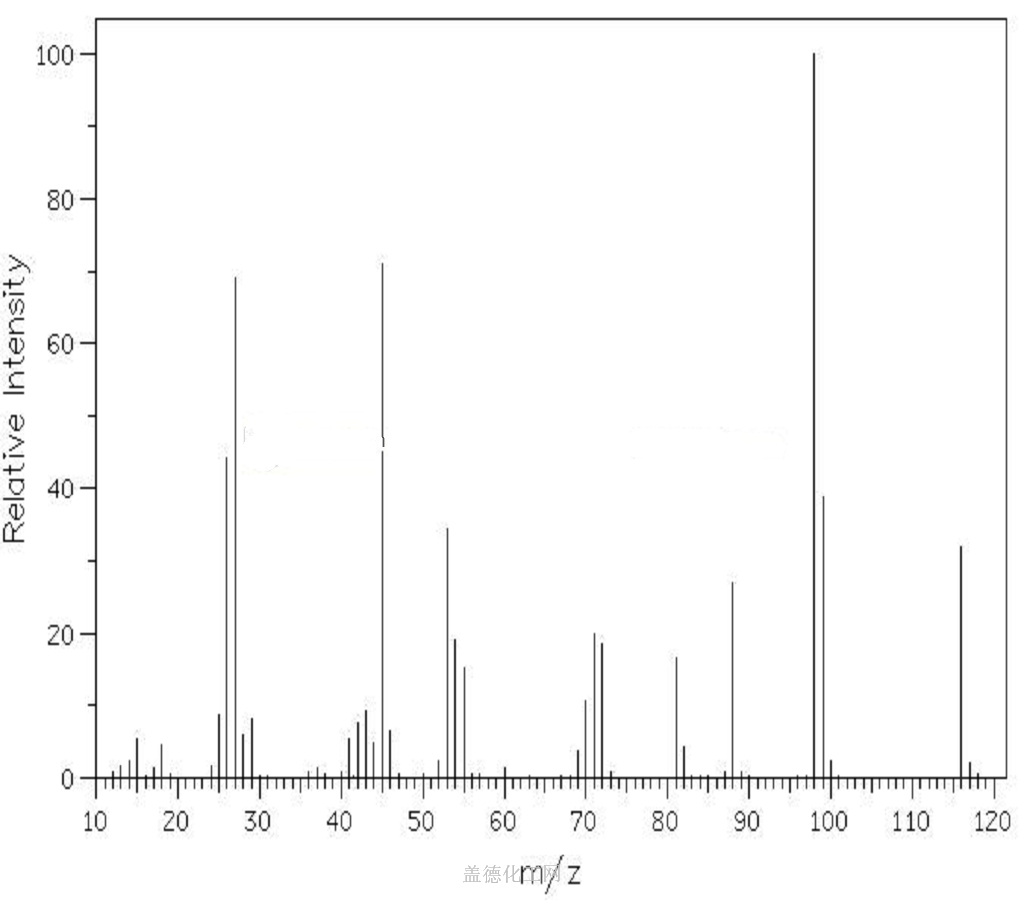
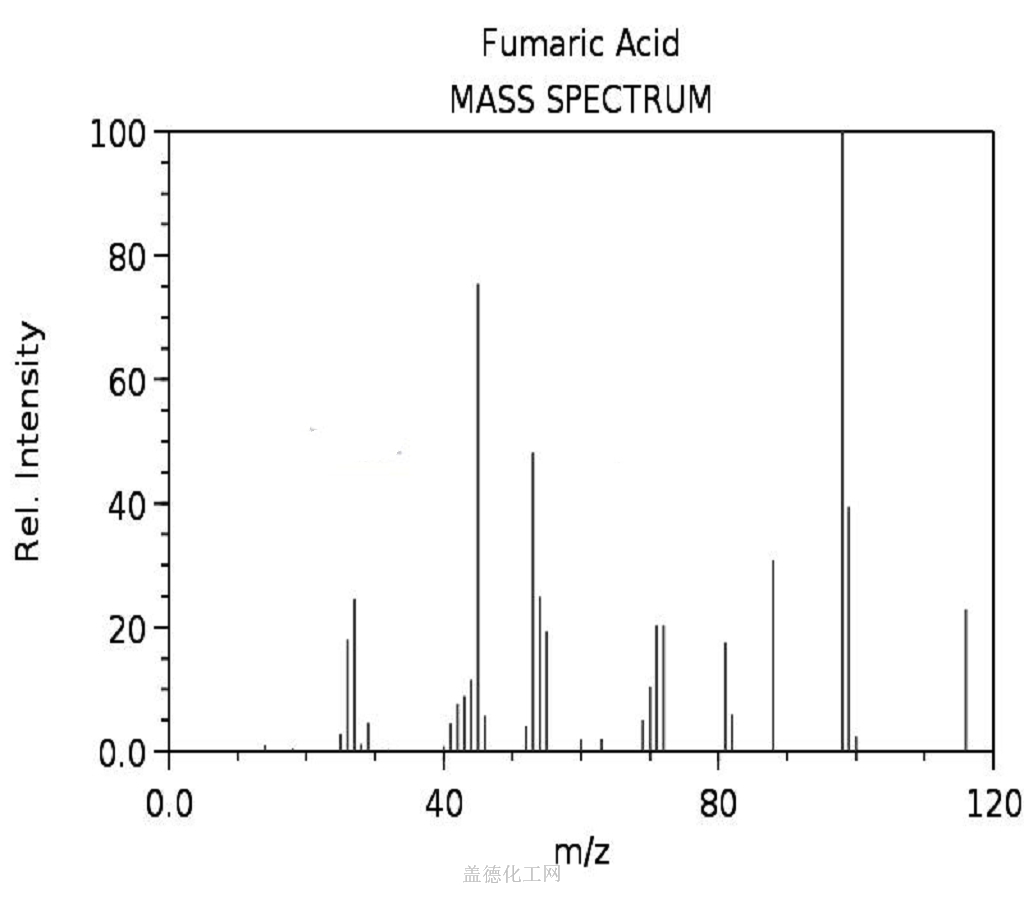
Fumaric acid is an important kind of organic chemical raw materials as well as the intermediate of fine chemical products. Meanwhile, it is also an important kind of derivatives of maleic anhydride, being widely used in food, coatings, resins and plasticizers. In the food industry, fumaric acid, used as souring agent, can be applied to soft drinks, western-class wine, cold drinks, fruit juice concentrate, canned fruit, pickles and ice cream. As an acidic substance used as solid beverage gas production agent, it has excellent bubble durability with delicate product organization.Fumaric acid has been used as a food acidulant since 1946. As a food additive, it is used as an acidity regulator and can be denoted by the E number E297. Chemically it is an unsaturated dicarbonic acid and is part of the citric acid cycle. Fumaric acid is a common food additive included in many processed foods to keep them stable and to add tartness. The substance has a more sour flavor than citric acid, another common food additive. Fumaric acid occurs naturally in fumitory, bolete mushrooms, lichen and Iceland moss. As an additive, fumaric acid is produced synthetically, mainly from malic acid from apples. Fumaric acid as an additive is regulated under the Codex Alimentarius General Standard for Food Additives (GSFA), a collection of internationally recognized standards.The U.S. Food and Drug Administration considers it safe.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. Safety and Handling
5. MSDS
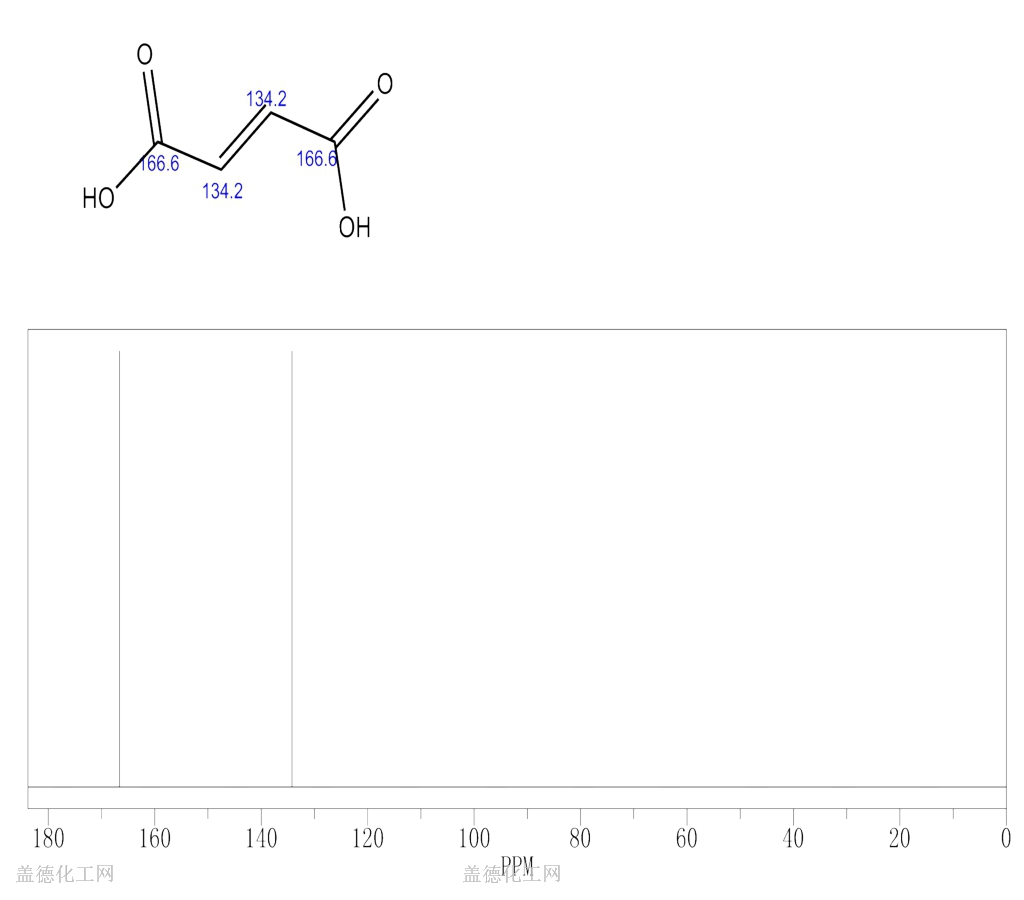
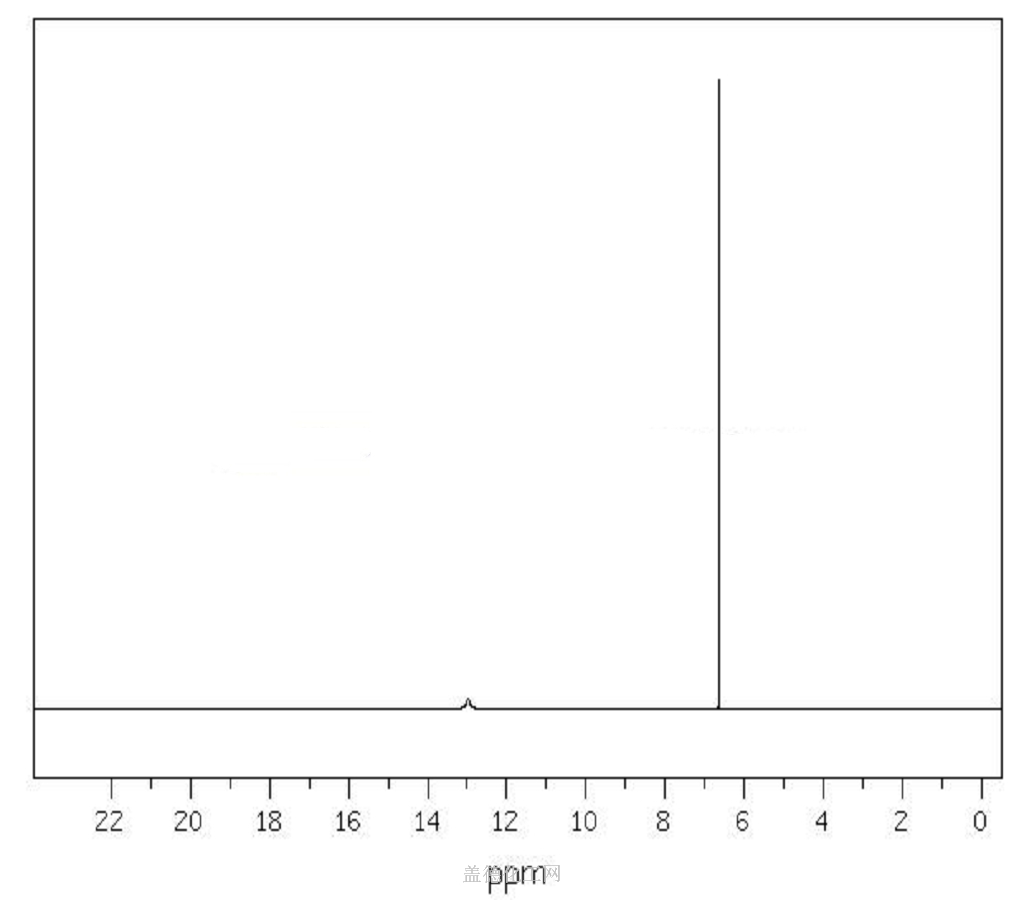
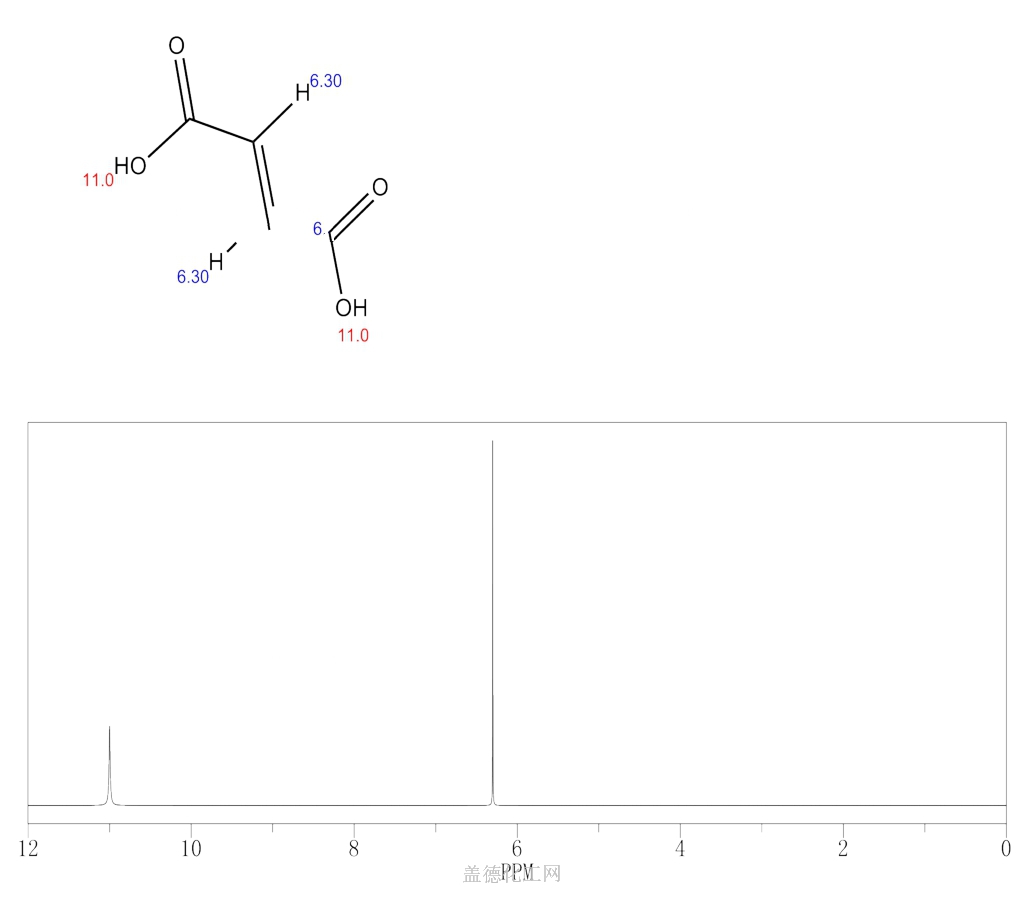
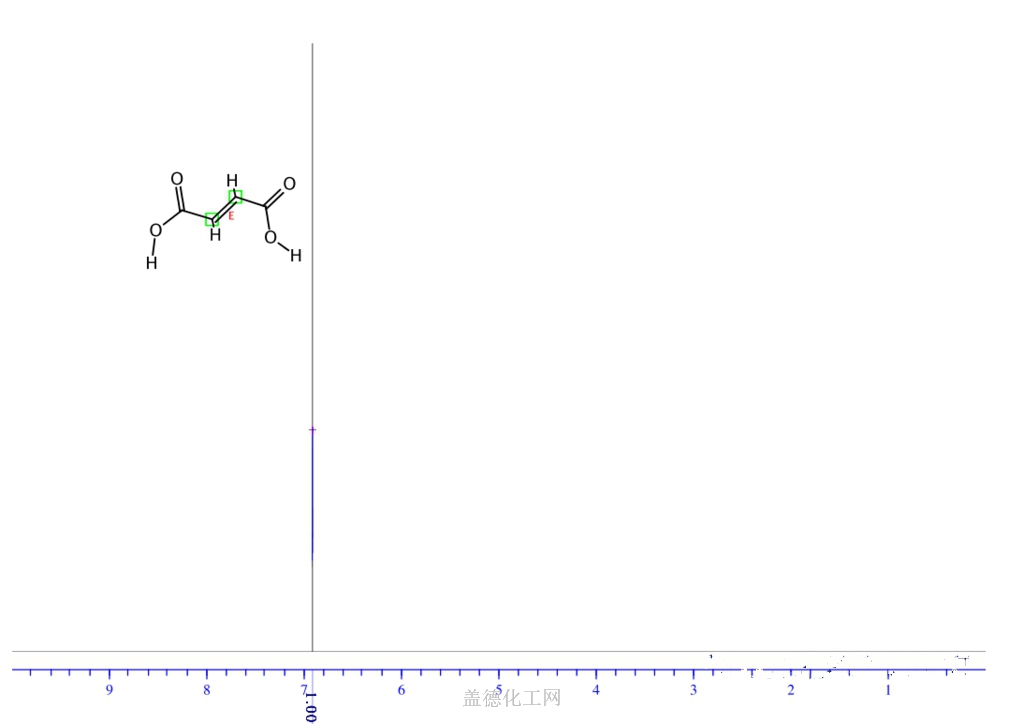
6. NMR Spectrum
7. Synthesis Route
8. Precursor and Product
9. Computed Properties
12. Related Questions
13. Realated Product Infomation

 EN
EN


 Xi
Xi