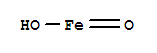Iron hydroxide oxide, with the chemical formula FeO(OH) and CAS registry number 20344-49-4, is a compound known for its various applications. This compound is commonly used as a pigment in paints and coatings due to its reddish-brown color. It is also used in the production of iron oxide pigments, which are widely used in the manufacturing of ceramics, plastics, and rubber. Iron hydroxide oxide is insoluble in water and is often used as a corrosion inhibitor in industrial processes. Additionally, it has been studied for its potential use in wastewater treatment due to its ability to adsorb heavy metals. Overall, iron hydroxide oxide is a versatile compound with important industrial applications.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. Safety and Handling
5. MSDS
6. Computed Properties
9. Related Questions
10. Realated Product Infomation

 EN
EN