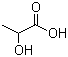
Lactic acid (2-hydroxypropionic acid, CH3-CHOH-COOH) is the most widelyoccurring organic acid in nature. Due to its chiral a-carbon atom, lactic acid (LA)has two enantiomeric forms. Of these, L-(+)-lactic acid is more important in food and pharmaceutical industries because humans have only L-lactatedehydrogenase. The chemical behavior of lactic acid is mostly determinedby the two functional groups. Besides the acidic character in aqueousmedium, the bifunctionality (a terminal carboxylic acid and a hydroxyl group)allows lactic acid molecules to form ‘‘interesters’’ such as the cyclic dimers, thetrimers, or longer lactic acid oligomers.After its first isolation by the Swedish chemist Scheel in 1780 from sour milk,lactic acid has been produced commercially since the 1880s in the United Statesand later in Europe. Worldwide, lactic acid production was approximately250,000 metric tons per year in 2012 and is expected to reach 330,000metric tons by the year 2015, with an average price of 1.25 US$ perkilogram in 2013 (food grade, 80–85 % purity). Approximately 85 % of the demand for LA is from the food industry. Theprimary use of lactic acid is as a pH-adjusting agent in the beverage sector and as apreservative in the food industry. It is included in the Generally Recognized asSafe (GRAS) by the U.S. Food and Drug Administration [158] as a food ingredientand was deemed safe by the European Food Safety Authority as well [159]. Theacceptable daily intake for LA was defined by the Joint FAO/WHO ExpertCommittee on Food Additives as ‘‘not limited,’’ and it is also supported by theScientific Committee of Food. In recent decades, the consumption of lactic acid due to its novel applicationshas grown quite rapidly, by 19 % per year. Nonfood use of lactic acid for polymer production contributes to this growth. Biodegradable polylactic acid isconsidered to be an environmentally friendly alternative to other plastics frompetroleum. It is used in various fields, including drug delivery systems,medical devices, fibers, and packaging materials. Lactic acid can be produced via chemical synthesis or carbohydrate fermentation.The chemical route has various issues, including toxic raw materials, lowconversion rates, and especially the inability to produce the optically pure isomer.Therefore, approximately 90 % of lactic acid worldwide is produced by biotechnologicalprocesses, namely fermentations using renewable resources, whichis relatively fast, economical, and able to supply selectively one or two stereoisomersof lactic acid.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. Safety and Handling
5. MSDS
6. NMR Spectrum
7. Synthesis Route
8. Precursor and Product
9. Computed Properties
12. Related Questions
13. Realated Product Infomation

 EN
EN

 T
T