-

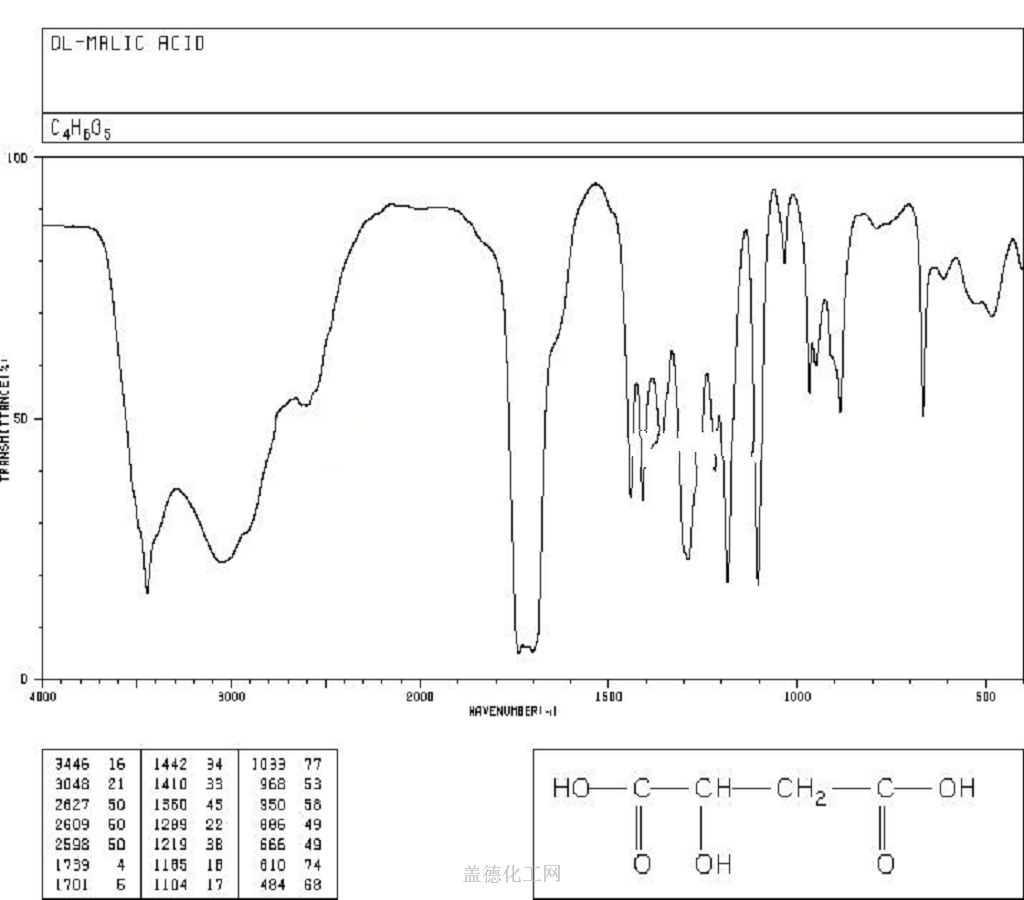
Malic acid
- CAS:6915-15-7
- MW:134.08744
- MF:C4H6O5
Malic acid, HOOCCH(OH).CH2COOH, also known as hydroxysuccinic acid, is a colorless solid. It is soluble in water and alcohol. Malic acid exists in two optically active forms and a racemic mixture. It is used in medicine and found in apples and other fruits.The naturally occuring isomer is the L-form which has been found in apples and many other fruits and plants. Selective α-amino protecting reagent for amino acid derivatives. Versatile synthon for the preparation of chiral compounds including κ-opioid rece
View more+
 EN
EN