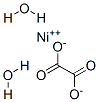
NICKEL OXALATE DIHYDRATE, with the chemical formula NiC2O4·2H2O and CAS registry number 6018-94-6, is a compound that consists of nickel ions bonded to oxalate ions and water molecules. It is a green crystalline solid that is soluble in water. Nickel oxalate dihydrate is commonly used as a precursor for the synthesis of other nickel compounds and as a catalyst in various chemical reactions. It is also used in the production of nickel-based pigments and as a source of nickel ions in electroplating processes. Nickel oxalate dihydrate has been studied for its potential applications in battery technology and as a catalyst for carbon dioxide reduction. It is important to note that the information provided here is based on the latest available data and may be subject to change as new research and discoveries are made.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. MSDS
5. Computed Properties
8. Realated Product Infomation

 EN
EN