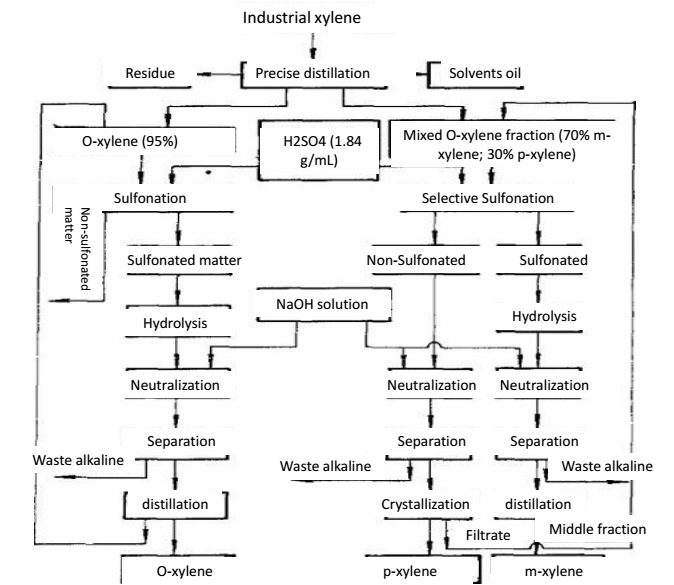
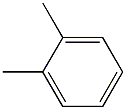
o-Xylene (CAS 95-47-6) is a colorless liquid with a sweet, aromatic odor. It has a basic structure consisting of a benzene ring with two methyl groups attached to it. This chemical is highly soluble in organic solvents such as ethanol and ether, but only slightly soluble in water. o-Xylene has a boiling point of 144.4°C and a melting point of -25.2°C. It is flammable and can form explosive mixtures with air. In terms of chemical properties, o-Xylene can undergo various reactions such as oxidation, halogenation, and nitration.
Applicable Fields
o-Xylene has several applications in different fields:
Chemical Industry: In the chemical industry, o-Xylene is used as a solvent for various purposes. It is commonly employed in the production of phthalic anhydride, which is a key ingredient in the manufacturing of plasticizers, polyester resins, and alkyd resins. The mechanism of action involves o-Xylene dissolving or dispersing other substances, allowing for efficient chemical reactions.
Paint and Coatings Industry: o-Xylene is also utilized in the paint and coatings industry as a solvent for paints, varnishes, and lacquers. It helps in achieving the desired consistency and viscosity of the products. The mechanism of action involves o-Xylene acting as a carrier for the pigments and binders, allowing for easy application and drying.
Adhesive Industry: In the adhesive industry, o-Xylene is used as a solvent in the production of various types of adhesives. It helps in dissolving the adhesive components and provides the necessary viscosity for application. The mechanism of action involves o-Xylene evaporating after application, allowing the adhesive to bond the surfaces together.
Storage Conditions
The storage conditions for o-Xylene are as follows: Store in a cool, well-ventilated area, away from ignition sources and heat. Keep the container tightly closed and upright to prevent leakage. Avoid direct sunlight and exposure to flames or sparks.

 EN
EN