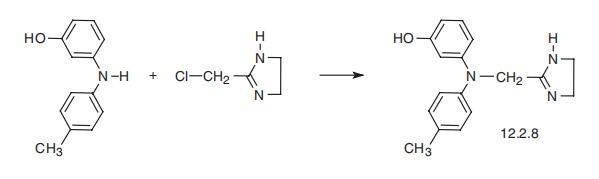
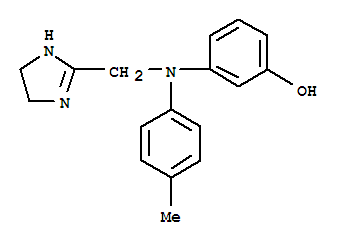
-

Phentolamine
- CAS:50-60-2
- MW:281.359
- MF:C17H19N3O
Phentolamine is an α-adrenergicreceptor antagonist approved for use by the U.S.Food and Drug Administration (FDA) in 1952. Approved uses of phentolamine currently include (1) diagnosis of pheochromocytoma,(2) treatment of hypertension in pheochromocytoma, and (3) prevention of tissue necrosis after norepinephrine extravasation. Anearly use of injectable phentolamine involved the management of impotence (erectile dysfunction).Phentolamine is a short-acting, competitive antagonist at peripheral o-adrenergic receptors. It antagonizes both a and ozreceptors,thus blocking the actions of the circulating catecholamines epinephrine and norepinephrine. Phentolamine also stimulates β-adrenergic receptors in the heart and lungs.
View more+
 EN
EN