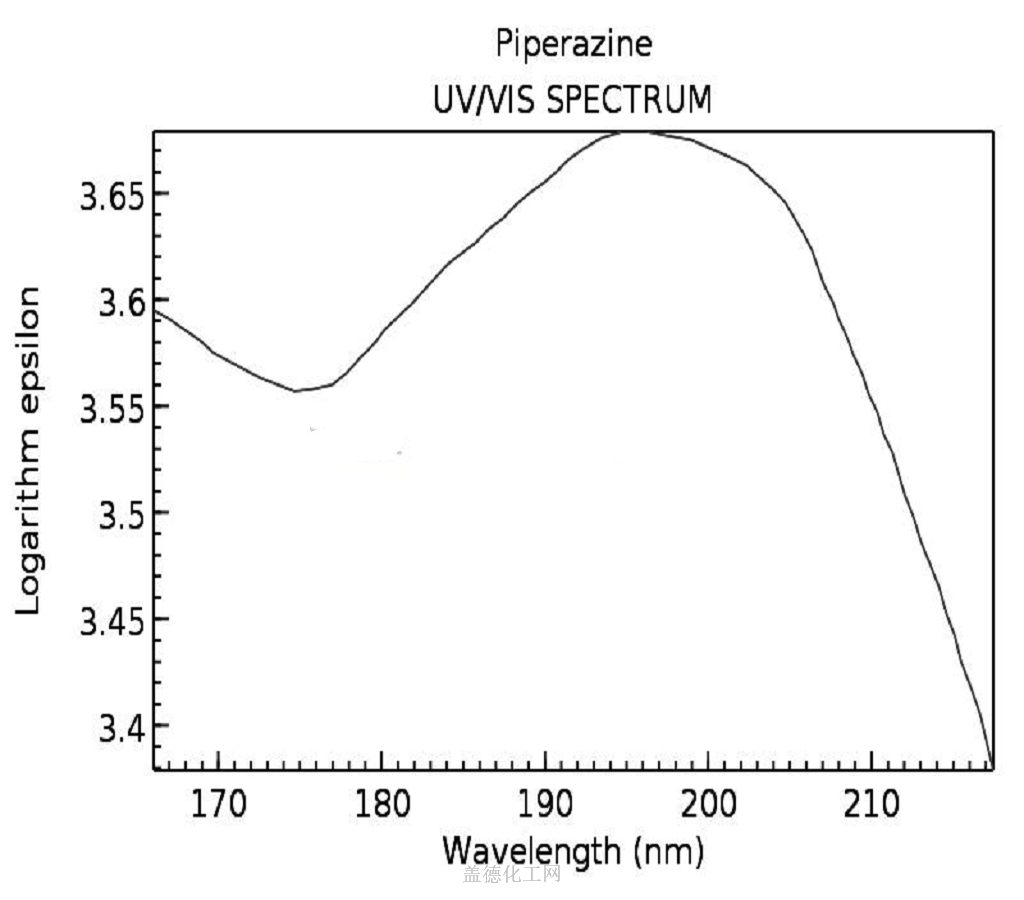
Piperazine (CAS 110-85-0) is a heterocyclic organic compound that appears as a colorless liquid with a strong odor. It has a basic structure consisting of a six-membered ring with two nitrogen atoms. Piperazine is highly soluble in water, and it is also soluble in alcohol and ether. It has a boiling point of 146-148°C and a melting point of -20°C. This chemical is commonly used in various industries due to its unique properties.
Applicable Fields
Piperazine has several applications in different fields:
Pharmaceutical Industry: In the pharmaceutical industry, piperazine is used as an intermediate in the synthesis of various drugs. It acts as a building block for the production of antihistamines, anthelmintics, and other medications. The mechanism of action of piperazine in these drugs varies depending on the specific compound.
Chemical Industry: Piperazine is also utilized in the chemical industry for the production of corrosion inhibitors, textile auxiliaries, and epoxy curing agents. Its mechanism of action in corrosion inhibitors involves forming a protective film on metal surfaces, preventing them from reacting with corrosive substances.
Animal Health: Piperazine is commonly used as an anthelmintic agent in veterinary medicine. It is effective against various parasites, including roundworms and pinworms, in animals. The mechanism of action involves paralyzing the parasites, making them easier to eliminate from the animal's system.
Storage Conditions
Piperazine should be stored in a cool and dry place, away from direct sunlight.
- 3.3 Potential Exposure
- (Piperazine): Primary irritant (w/o allergic reaction),
- 3.4 Purification Methods
- Piperazine crystallises from EtOH or anhydrous *benzene and is dried at 0.01mm. It can be sublimed under vacuum and purified by zone melting. The hydrochloride has m 172-174o (from EtOH), and the dihydrochloride crystallises from aqueous EtOH and has m 318-320o (dec, sublimes at 295-315o). The picrate has m ~200o, and the picrolonate crystallises from dimethylformamide ( m 259-261o). [Beilstein 23 H 4, 23 I 4, 23 II 3, 23 III/IV 15, 23/1 V 30.]
- 3.5 Shipping
- UN2579 Piperazine, Hazard class: 8; Labels: 8-Corrosive material.
- 3.6 Usage
- Labelled Piperazine
4. Safety and Handling
- 4.1 Exposure Standards and Regulations
- The Approved Drug Products with Therapeutic Equivalence Evaluations List identifies currently marketed discontinued drug products, incl piperazine citrate, approved on the basis of safety and effectiveness by FDA under sections 505 of the Federal Food, Drug, and Cosmetic Act. /piperazine citrate/
- 4.2 Octanol/Water Partition Coefficient
- log Kow = -1.50
- 4.3 Fire Hazard
- Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
- 4.4 Other Preventative Measures
- If material not on fire and not involved in fire: Keep sparks, flames, and other sources of ignition away. Keep material out of water sources and sewers. Build dikes to contain flow as necessary.
Personnel protection: Avoid breathing vapors. Keep upwind. ... Avoid bodily contact with the material. ... Do not handle broken packages unless wearing appropriate personal protective equipment. Wash away any material which may have contacted the body with copious amounts of water or soap and water. If contact with the material anticipated, wear appropriate chemical protective clothing.
The worker should immediately wash the skin when it becomes contaminated.
Work clothing that becomes wet or significantly contaminated should be removed and replaced.
Workers whose clothing may have become contaminated should change into uncontaminated clothing before leaving the work premises.
SRP: Contaminated protective clothing should be segregated in such a manner so that there is no direct personal contact by personnel who handle, dispose, or clean the clothing. Quality assurance to ascertain the completeness of the cleaning procedures should be implemented before the decontaminated protective clothing is returned for reuse by the workers. Contaminated clothing should not be taken home at end of shift, but should remain at employee's place of work for cleaning.
- 4.5 Cleanup Methods
- Evacuate persons not wearing protective equipment from area of spill or leak until clean-up is complete. Remove all ignition sources. Collect powdered material in the most convenient and safe manner and deposit in sealed containers. Ventilate area after clean-up is complete. It may be necessary to contain and dispose of this chemical as a hazardous waste. If material or contaminated runoff enters waterways, notify downstream users of potentially contaminated waters. Contact your Department of Environmental Protection or your regional office of the federal EPA for specific recommendations. If employees are required to clean-up spills, they must be properly trained and equipped. OSHA 1910.120(q) may be applicable.
- 4.6 DisposalMethods
- SRP: At the time of review, criteria for land treatment or burial (sanitary landfill) disposal practices are subject to significant revision. Prior to implementing land disposal of waste residue (including waste sludge), consult with environmental regulatory agencies for guidance on acceptable disposal practices.
- 4.7 DOT Emergency Guidelines
- /GUIDE 153: SUBSTANCES - TOXIC AND/OR CORROSIVE (COMBUSTIBLE)/ Health: TOXIC; inhalation, ingestion, or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
/GUIDE 153: SUBSTANCES - TOXIC AND/OR CORROSIVE (COMBUSTIBLE)/ Fire or Explosion: Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors, and sewers explosion hazards. Those substances designated with a "P" may polymerize explosively when heated or involved in a fire. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
/GUIDE 153: SUBSTANCES - TOXIC AND/OR CORROSIVE (COMBUSTIBLE)/ Public Safety: CALL Emergency Response Telephone Number ... . As an immediate precautionary measure, isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids. Keep unauthorized personnel away. Stay upwind. Keep out of low areas. Ventilate enclosed areas.
/GUIDE 153: SUBSTANCES - TOXIC AND/OR CORROSIVE (COMBUSTIBLE)/ Protective Clothing: Wear positive pressure self-contained breathing apparatus (SCBA). Wear chemical protective clothing that is specifically recommended by the manufacturer. It may provide little or no thermal protection. Structural firefighters' protective clothing provides limited protection in fire situations ONLY; it is not effective in spill situations where direct contact with the substance is possible.
/GUIDE 153: SUBSTANCES - TOXIC AND/OR CORROSIVE (COMBUSTIBLE)/ Evacuation: ... Fire: If tank, rail car or tank truck is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions.
/GUIDE 153: SUBSTANCES - TOXIC AND/OR CORROSIVE (COMBUSTIBLE)/ Fire: Small fires: Dry chemical, CO2 or water spray. Large fires: Dry chemical, CO2, alcohol-resistant foam or water spray. Move containers from fire area if you can do it without risk. Dike fire control water for later disposal; do not scatter the material. Fire involving tanks or car/trailer loads: Fight fire from maximum distance or use unmanned hose holders or monitor nozzles. Do not get water inside containers. Cool containers with flooding quantities of water until well after fire is out. Withdraw immediately in case of rising sound from venting safety devices or discoloration of tank. ALWAYS stay away from tanks engulfed in fire.
/GUIDE 153: SUBSTANCES - TOXIC AND/OR CORROSIVE (COMBUSTIBLE)/ Spill or Leak: ELIMINATE all ignition sources (no smoking, flares, sparks or flames in immediate area). Do not touch damaged containers or spilled material unless wearing appropriate protective clothing. Stop leak if you can do it without risk. Prevent entry into waterways, sewers, basements or confined areas. Absorb or cover with dry earth, sand or other non-combustible material and transfer to containers. DO NOT GET WATER INSIDE CONTAINERS.
/GUIDE 153: SUBSTANCES - TOXIC AND/OR CORROSIVE (COMBUSTIBLE)/ First Aid: Move victim to fresh air. Call 911 or emergency medical service. Give artificial respiration if victim is not breathing. Do not use mouth-to-mouth method if victim ingested or inhaled the substance; give artificial respiration with the aid of a pocket mask equipped with a one-way valve or other proper respiratory medical device. Administer oxygen if breathing is difficult. Remove and isolate contaminated clothing and shoes. In case of contact with substance, immediately flush skin or eyes with running water for at least 20 minutes. For minor skin contact, avoid spreading material on unaffected skin. Keep victim warm and quiet. Effects of exposure (inhalation, ingestion or skin contact) to substance may be delayed. Ensure that medical personnel are aware of the material(s) involved and take precautions to protect themselves.
- 4.8 Fire Fighting Procedures
- Piperazine is a combustible liquid. Use dry chemical, carbon dioxide, water spray, or alcohol foam extinguishers. Piperazine may burn, but does not readily ignite. Extinguish fire using an agent suitable for type of surrounding fire. Poisonous gases including nitrogen oxides and hydrogen chloride (hydrochloride) are produced in fire. If material or contaminated runoff enters waterways, notify downstream users of potentially contaminated waters. Notify local health and fire officials and pollution control agencies. Containers may explode in fire. From a secure, explosion-proof location, use water spray to cool exposed containers. If cooling streams are ineffective (venting sound increases in volume and pitch, tank discolors, or shows any signs of deforming), withdraw immediately to a secure position. If employees are expected to fight fires, they must be trained and equipped in OSHA 1910.156.
If material on fire or involved in fire: Extinguish fire using agent suitable for type of surrounding fire. (Material itself does not burn or burns with difficulty.) Use water in flooding quantities as fog. Apply water from as far a distance as possible. Use water spray to knock-down vapors.
- 4.9 FirePotential
- Combustible when exposed to heat or flame.
- 4.10 Safety Profile
- Moderately toxic byingestion, skin contact, intravenous, andsubcutaneous routes. Mildly toxic byinhalation. A skin and severe eye irritant.Excessive absorption can cause urticaria,vomiting, diarrhea, blurred vision, andweakness. Combustible when exposed toheat or flame; can react vigorously withoxidizing materials. Explodes on contactwith dicyanofurazan. To fight fire, usealcohol foam, mist, dry chemical, waterspray. When heated to decomposition itemits highly toxic fumes of NOx.
- 4.11 Formulations/Preparations
- U.S.- Not commercially available. Canada-2 grams per packet (Rx) (Entacyl (sodium
U.S.- Not commercially available. Canada- 600 mg per 5 mL (Rx) (Entacyl (sodium
U.S.- 250 mg (hexahydrate) (275.75 mg piperazine citrate anhydrous) (Rx) (GENERIC). Canada- Not commercially available. /Piperazine citrate Tablets USP/
Piperazine salts are available as tablets & wafers, each containing 500 mg, & as syrups & suspensions containing 100 mg/mL, calculated as hexahydrate. ... Piperazine citrate, USP, and piperazine phosphate, NF, are official preparations. Trade names include Antepar (citrate & phosphate), Multifuge citrate and Pipizan citrate.
Piperazine calcium edathamil (Perin) syrup, 500 mg (of piperazine hexahydrate) per 5 mL. Tablets, 500 mg (of piperazine hexahydrate).
Piperazine gluconate (vermizine gluconate) syrup, 500 mg (... Hexahydrate) per 5 mL.
VET: Piperazine hexahydrate 43%, adipate 37%, citrate 40%, dihydrochloride 53% & phosphate 42.5% are popular in drinking water medication; piperazine phosphate 42.5% & dihydrochloride 53% are popular in dry feed or drinking water medication.
VET: Other less commonly used forms are dithiocarbamate, gluconate, glycolylarsanilate, monochloride, & sulfate. These above forms are considered equally effective in terms of their piperazine base ... . A number of complexes of mixtures with other drugs have been made to theoretically improve drug's activity.
Pipertab 2: Composition: each tablet contains: piperazine dihydrochloride equivalent to 50 mg piperazine base. ... Available in 500 tablet bottles. Pipertab 10: Composition: each tablet contains: piperazine dihydrochloride equivalent to 250 mg piperazine base. /Piperazine Dihydrochloride/
52% piperazine base grades /PIPERAZINE HYDROCHLORIDE/
- 4.12 Incompatibilities
- Aqueous solution is a strong base. Violent reaction with strong oxidizers and dicyanofurazan. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitrogen compounds, carbon tetrachloride. Attacks aluminum, copper, nickel, magnesium and zinc. Piperazine Preparation Products And Raw materials Raw materials
- 4.13 Protective Equipment and Clothing
- Wear appropriate personal protective clothing to prevent skin contact.
Wear appropriate eye protection to prevent eye contact.
Eyewash fountains should be provided in areas where there is any possibility that workers could be exposed to the substance; this is irrespective of the recommendation involving the wearing of eye protection.
Facilities for quickly drenching the body should be provided within the immediate work area for emergency use where there is a possibility of exposure. [Note: It is intended that these facilities provide a sufficient quantity or flow of water to quickly remove the substance from any body areas likely to be exposed. The actual determination of what constitutes an adequate quick drench facility depends on the specific circumstances. In certain instances, a deluge shower should be readily available, whereas in others, the availability of water from a sink or hose could be considered adequate.]
- 4.14 Reactivities and Incompatibilities
- Violent reaction with strong oxidizers and dicyanofurazan. Incompatible with nitrogen compounds, carbon tetrachloride. Attacks aluminum, copper, nickel, magnesium, and zinc.
- 4.15 Report
-
Reported in EPA TSCA Inventory.
- 4.16 Safety
-
Hazard Codes:? C
C
Risk Statements: 34-42/43-52/53?
R34:Causes burns.?
R42/43:May cause sensitization by inhalation and skin contact.?
R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Statements: 22-26-36/37/39-45-61?
S22:Do not breathe dust.?
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.?
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.?
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)?
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
RIDADR: UN 2579 8/PG 3
WGK Germany: 1
RTECS: TK7800000
F: 3-8-23
Hazard Note: Harmful/Corrosive
HazardClass: 8
PackingGroup: III
Moderately toxic by ingestion, skin contact, intravenous, and subcutaneous routes. Mildly toxic by inhalation. A skin and severe eye irritant. Excessive absorption can cause urticaria, vomiting, diarrhea, blurred vision, and weakness. Combustible when exposed to heat or flame; can react vigorously with oxidizing materials. Explodes on contact with dicyanofurazan. To fight fire, use alcohol foam, mist, dry chemical, water spray. When heated to decomposition it emits highly toxic fumes of NOx.
- 4.17 Sensitive
- Air Sensitive & Hygroscopic
- 4.18 Specification
-
?Piperazine (CAS NO.110-85-0) is also named as 1,4-Diazacyclohexane ; 1,4-Diethylenediamine ; 1,4-Piperazine ; 5-23-01-00030 (Beilstein Handbook Reference) ; Antiren ; Asca-Trol No. 3 ; BRN 0102555 ; CCRIS 5950 ; Diethylenediamine ; Diethyleneimine ; Dispermine ; Entacyl ; Eraverm ; Eraverm (VAN) ; HSDB 1093 ; Hexahydro-1,4-diazine ; Hexahydropyrazine ; Lumbrical ; NSC 474 ; Piperazidine ; Piperazin ; Piperazin [German] ; Piperazine, anhydrous ; Pipersol ; Pyrazine hexahydride ; Pyrazine, hexahydro- ; UNII-1RTM4PAL0V ; Uvilon ; Vermex ; Worm-A-Ton ; Wurmirazin?.?Piperazine (CAS NO.110-85-0) is?needle-like white or colorless crystals. It is very corrosive to skin, eyes and mucous membranes. Solid?of Piperazine turns dark when exposed to light. It is flammable and soluble in water.?Piperazine? neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Absorbs carbon dioxide from the air, which can cause dry crystals to seem to melt. May generate hydrogen, a flammable gas, in combination with strong reducing agents such as hydrides.?Inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. It may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.?
- 4.19 Toxicity
-
| Organism |
Test Type |
Route |
Reported Dose (Normalized Dose) |
Effect |
Source |
| child |
TDLo |
oral |
75mg/kg (75mg/kg) |
BEHAVIORAL: SLEEP
GASTROINTESTINAL: NAUSEA OR VOMITING |
"Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 478, 1969. |
| mouse |
LC50 |
inhalation |
5400mg/m3/2H (5400mg/m3) |
BEHAVIORAL: EXCITEMENT
BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY)
BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) |
Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 15, Pg. 116, 1979. |
| mouse |
LD50 |
intraperitoneal |
1900mg/kg (1900mg/kg) |
? |
Progress in Biochemical Pharmacology. Vol. 1, Pg. 542, 1965. |
| mouse |
LD50 |
intravenous |
1180mg/kg (1180mg/kg) |
? |
Drugs in Japan Vol. 6, Pg. 635, 1982. |
| mouse |
LD50 |
oral |
600mg/kg (600mg/kg) |
? |
Bollettino Chimico Farmaceutico. Vol. 103, Pg. 414, 1964. |
| rabbit |
LD50 |
skin |
4mL/kg (4mL/kg) |
? |
Union Carbide Data Sheet. Vol. 7/16/1965, |
| rat |
LD50 |
intramuscular |
> 2500mg/kg (2500mg/kg) |
? |
Drugs in Japan Vol. 6, Pg. 635, 1982. |
| rat |
LD50 |
intravenous |
1340mg/kg (1340mg/kg) |
? |
Drugs in Japan Vol. 6, Pg. 635, 1982. |
| rat |
LD50 |
oral |
1900mg/kg (1900mg/kg) |
BEHAVIORAL: EXCITEMENT
BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY)
BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) |
Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 15, Pg. 116, 1979. |
| rat |
LD50 |
subcutaneous |
3700mg/kg (3700mg/kg) |
? |
Drugs in Japan Vol. 6, Pg. 635, 1982. |
5. MSDS
2.Hazard identification
2.1 Classification of the substance or mixture
no data available
2.2 GHS label elements, including precautionary statements
| Pictogram(s) | no data available |
| Signal word | no data available |
| Hazard statement(s) | no data available |
| Precautionary statement(s) | |
| Prevention | no data available |
| Response | no data available |
| Storage | no data available |
| Disposal | no data available |
2.3 Other hazards which do not result in classification
no data available
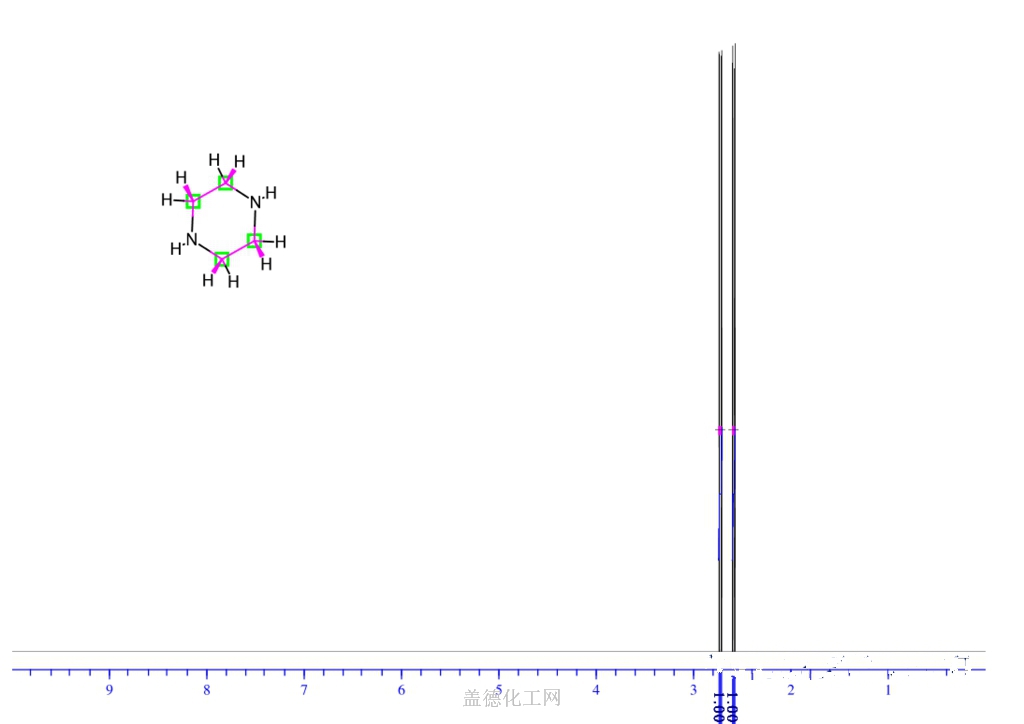
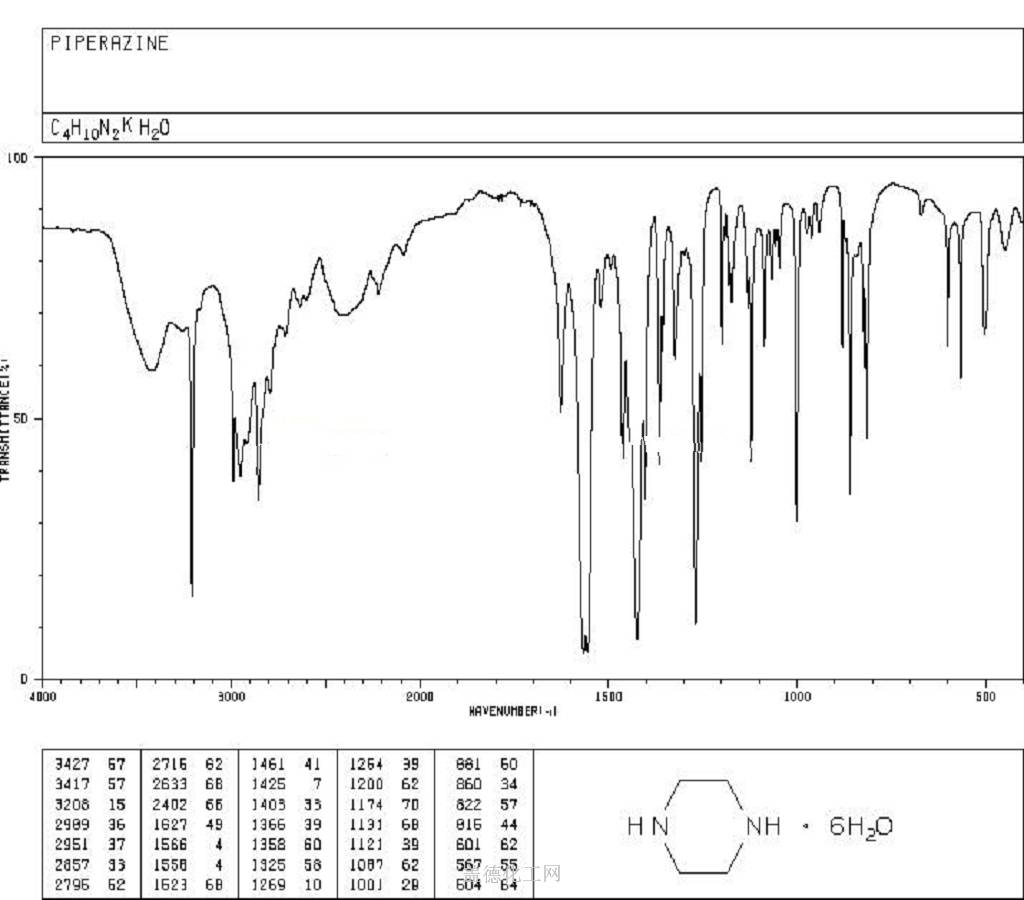
6. NMR Spectrum
1H NMR : parameter in CDCl3
1H NMR : parameter in D2O
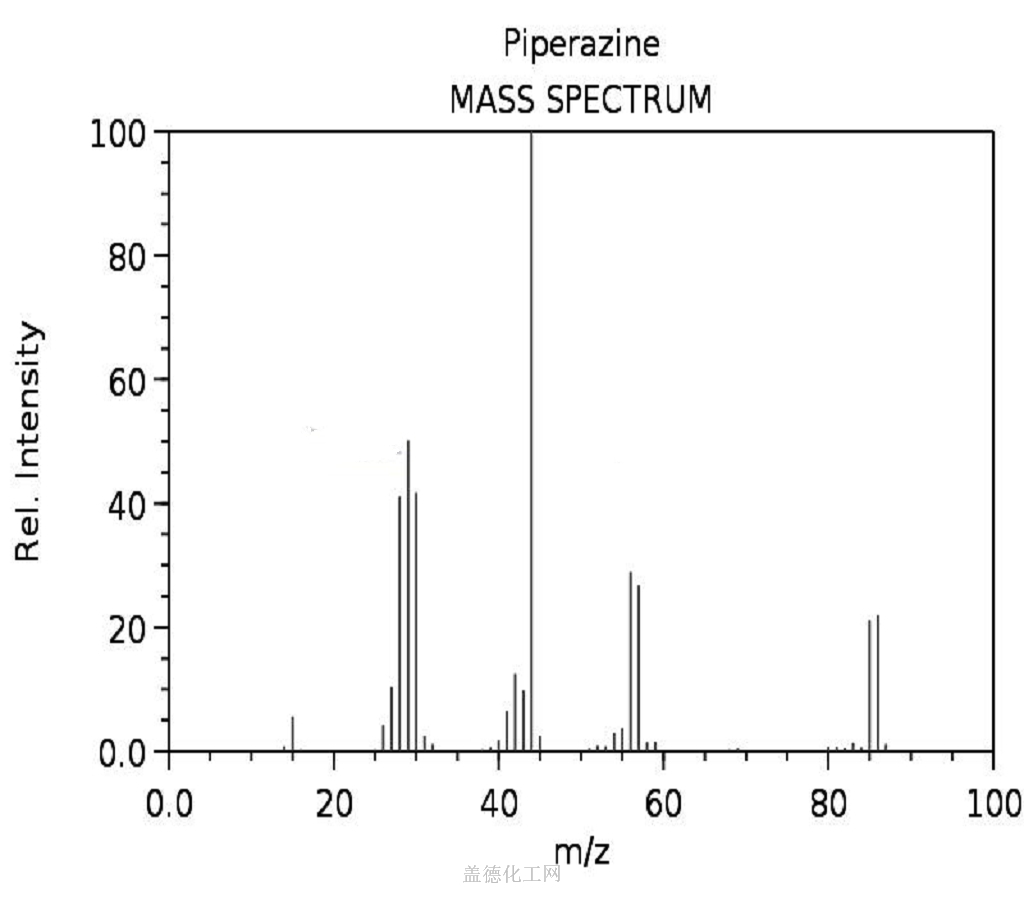
Mass spectrum (electron ionization)
7. Computed Properties
- Molecular Weight:86.13560g/mol
- Molecular Formula:C4H10N2
- Compound Is Canonicalized:True
- XLogP3-AA:86.084398327
- Monoisotopic Mass:86.084398327
- Complexity:26.5
- Rotatable Bond Count:0
- Hydrogen Bond Donor Count:2
- Hydrogen Bond Acceptor Count:2
- Topological Polar Surface Area:24.1
- Heavy Atom Count::6
- Defined Atom Stereocenter Count:0
- Undefined Atom Stereocenter Count:0
- Defined Bond Stereocenter Count:0
- Undefined Bond Stereocenter Count:0
- Isotope Atom Count:0
- Covalently-Bonded Unit Count:1
- CACTVS Substructure Key Fingerprint:AAADccBjAAAAAAAAAAAAAAAAAAAAAAAAAAAsAAAAAAAAAAAAAAAAHAAQAAAAAADBAAQAAALAAAAAAAAAAAAAAAAAAAAAAIAIAAAAQAAAAAAQAAAAEACAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA==
8.Other Information
- Usage
- Piperazine acts as an intermediate for pharmaceuticals, polymers, dyes, corrosion inhibitors, rubber accelerators and surfactants. It is used as a raw material in the manufacture of plastics, polyamide resins, urethane and brake fluid. It is also used for carbondioxide and hydrogen sulfide gas scrubbing mediums. Furthermore, it serves as an epoxy curing agent.
- Merck
- 14,7464
- BRN
- 102555
- Important pharmaceutical intermediates
- Piperazine is an important pharmaceutical intermediate, is mainly used for the production of anthelmintic piperazine phosphate, piperazine citrate and fluphenazine, strong pain, rifampicin, adipic acid piperazine, piperazine guanidine methyl tetracycline, quinoline piperazine phosphate, piperazine thiazole nitrate, enoxacin, hydroxyzine hydrochloride, trifluoperazine, diethylcarbamazine citrate, cinnarizine, flunarizine, decloxizine strong carbamazepine, prednisolone sodium phosphate, dexamethasone sodium phosphate, PPA, norfloxacin, ciprofloxacin, easy to cough piperazine, a piperazine Lee vancomycin, trimethoprim-triazine and other drugs. It is Also used for the production of surfactants products such as wetting agents, emulsifying agents,and dispersing agents ,and the production of plastic additives such as antioxidants, preservatives, stabilizers and rubber additives. It is derived from Dichloroethane by alcohol solution of ammonia.
![The structural formula of piperazine]()
Figure 1 The structural formula of piperazine.
- Pharmacology and mechanism of action
- Piperazine is a heterocyclic organic base widely used as an anthelminthic. It was originally developed for the treatment of gout. Its first successful use in helminthiasis was reported by Mouriquand et al. in 1951 [1]. Presently the drug is used in the treatment of infections caused by Ascaris lumbricoides and Enterobius vermicularis.
The drug causes flaccid paralysis in susceptible worms and the parasites lose their attachment to the intestinal wall, and are swept away by the normal bowel peristalsis. The biochemical mechanism behind this action is uncertain. Piperazine causes hyperpolarization of the Ascaris muscle rendering it unresponsive to acetylcholine [2].
- Indications
- Treatment of infections due to Ascaris lumbricoides and Enterobius vermicularis. When cost and availability are not a consideration, safer and more effective drugs such as mebendazole or albendazole should be used instead.
- Side effects
- Side effects commonly encountered with the recommended doses of piperazine are nausea, vomiting, abdominal cramps and diarrhoea which are usually mild and self-limiting. Although absolute incidence is unknown, severe side effects reported in the literature are rare. They can be classified into:
1. Allergic reactions such as urticaria, exantema, hypersensitivity, lacrimation, rhinorrea, productive cough, and bronchospasm[3,4].
2. Neuro-psychological reactions[5-11]:
(a) cerebral type such as vertigo, dizziness, tremor, incoordination, ataxia and hypotonia with EEG changes;
(b) psychic type such as depersonalization, hallucination and paranoic reactions;
(c) miscellaneous such as headache, visual disturbances, somnolence, coma and an increase in the number of petit mal attacks.
Neuro-psychological reactions are rare. Most cases reported concern children with pre disposing factors like neurological symptoms, renal diseases or those who have been treated with high doses of piperazine.
One case of haemolytic anaemia in a patient with G6PD deficiency [12], and one case of toxic hepatitis[13] have also been reported. However, no causal relationships can be established from these cases.
Nitrosation of piperazine to the potential carcinogen N-mononitrosopiperazine in the stomach of patients treated with normal therapeutic doses has been reported[14]. However, carcinogenicity related to the use of piperazine has not been reported despite the use of the drug over many years. In any case, this is unlikely to have any clinical implications with the short treatment period of nematodes.
- Contraindications and precautions
- Piperazine should not be given to patients with hypersensitivity or with neurological diseases,especially epileptic patients.
- Interactions
- In rats and mice, piperazine 1–5 g/kg subcutaneously, potentiates the side effects of chlorpromazine [15]. However, this is unlikely to have any clinical significance. Piperazine is antagonistic to pyrantel, bephenium and levamisole , but no potential clinical interactions have been reported.
- Preparations
- Several preparations, apart from the one mentioned below, containing various piperazine salts are available.
? Antepar? (Wellcome). Oral suspension 150 mg piperazine hexahydrate/ml. Tablets 500 mg piperazine hexahydrate.
- Acute oral toxicity
- rat LD50: 1900 mg/kg; Oral-Mouse LD50: 600 mg/kg
- Data Skin irritation
- rabbit 500 mg Mild; Eyes-rabbit 0.25 mg/24 hours of severe
- References
- 1. Mouriquand G, Roman E, Coisnard J (1951). Essai de traitement de l’oxyurose par la piperazine. J Méd Lyon, 32, 189–195.
2. del Castillo J, De Mello WC, Morales T (1964). Mechanism of the paralysing action of piperazine on Ascaris muscle. Br J Pharmacol, 22, 463–477.
3. Macmillan AL (1973). Generalized pustular drug rash. Dermatologia, 146, 285–291.
4. McCullagh SF (1968). Allergenicity of piperazine: a study in environmental aetiology. Br J Ind Med, 25, 319–325.
5. Belloni C, Rizzoni G (1967). Neurotoxic side-effects of piperazine. Lancet, ii, 369.
6. Berger JR, Globus M, Melamed E (1979). Acute transitory cerebellar dysfunction associated with piperazine adipate. Arch Neurol, 36, 180–181.
7. Bomb RS, Bedi HK (1976). Neurotoxic side-effects of piperazine. Trans R Soc Trop Med Hyg, 70, 358.
8. Gupta SR (1976). Piperazine neurotoxicity and psychological reaction. J Ind Med Ass, 66, 33–34.
9. Parsons AC (1971). Piperazine neurotoxicity. ‘Worm wobble’. BMJ, 4, 790–792.
10. Vallat JN, Vallat JM, Texier J, Léger J (1972). Les signes neurologiques d’intoxication par la piperazine. Bordeaux Médicale, 5, 394–400.
11. Nickey LN (1966). Possible precipitation of petit mal seizures with piperazine citrate. J Am Med Ass, 195, 193–194.
12. Buchanan N, Cassel R, Jenkins T (1971). G-6-PD deficiency and piperazine. BMJ, 2, 110.
13. Hamlyn AN, Morris JS, Sarkany I, Sherlock S (1976). Piperazine hepatitis. Gastroenterology, 70, 1144–1147.
14. Bellander T, sterdahl B-G, Hagmar L (1985). Formation of N-mononitrosopiperazine in the stomach and its excretion in the urine after oral intake of piperazine. Toxicol Appl Pharmacol, 80, 193–198.
15. Sturman G (1973). Interaction between piperazine and chlorpromazine. Br J Pharmacol, 50, 153–155.
- Flammability and hazardous characteristics
- Combustible; decomposition of toxic nitric oxide gas in case of thermal
- Storage characteristics
- Treasury ventilation low-temperature drying; and stored separately from acid
- Extinguishing agent
- Water spray, dry powder, carbon dioxide, alcohol-resistant foam
- Professional standards
- TWA 1 mg/m3; STEL 5 mg/m
- Description
- Piperazine is contained in pyrazinobutazone, an equimolecular sah of piperazine and phenylbutazone. Among occupational cases, most were reported in the pharmaceutical industry or laboratory, in nurses and in veterinarians.
- Description
- Piperazine (Item No. 24019) is an analytical reference standard categorized as a piperazine. This product is intended for research and forensic applications.
- Chemical Properties
- Colorless to yellow solid; salty taste.
- Chemical Properties
- Piperazine is white to cream-colored needles or powder. Characteristic ammonia-like odor. Combustible solids that do not easily ignite.
- Uses
- Labelled Piperazine
- Uses
- keratolytic, antiseborheic
- Uses
- Piperazine is used as an intermediate in themanufacture of dyes, pharmaceuticals, polymers,surfactants, and rubber accelerators.
- Indications
- Piperazine (Vermizine) contains a heterocyclic ring that lacks a carboxyl group. It acts on the musculature of the helminths to cause reversible flaccid paralysis mediated by chloride-dependent hyperpolarization of the muscle membrane. This results in expulsion of the worm. Piperazine acts as an agonist at gated chloride channels on the parasite muscle.
Piperazine has been used with success to treat A. lumbricoides and E. vermicularis infections, although mebendazole is now the agent of choice. Piperazine is administered orally and is readily absorbed from the intestinal tract. Most of the drug is excreted in the urine within 24 hours.
Piperazine is an appropriate alternative to mebendazole for the treatment of ascariasis, especially in the presence of intestinal or biliary obstruction. Cure rates of more than 80% are obtained following a 2-day regimen.
Side effects occasionally include gastrointestinal distress, urticaria, and dizziness. Neurological symptoms of ataxia, hypotonia, visual disturbances, and exacerbations of epilepsy can occur in patients with preexisting renal insufficiency. It should not be used in pregnant women because of the formation of a potentially carcinogenic and teratogenic nitrosamine metabolite. Concomitant use of piperazine and chlorpromazine or pyrantel should be avoided.
- Definition
- ChEBI: An azacycloalkane that consists of a six-membered ring containing two nitrogen atoms at opposite positions.
- Brand name
- Pincets (Marion Merrell Dow); Pinsirup (Marion Merrell Dow);Adelmintex;Adipalis;Adipalit;Adiver;Ancaris thenium;Ancazine;Antelmina;Antepar (b-w);Anterobius;Anthalazine;Anthelmina;Anticucs;Antivermine;Ascalix;Ascarinex;Ascarivet;Asca-trol no.3;Asepar;Askaripar;Averamexan;Bel-zine;Bioxurin;B-piperazine;Brirel;Candizine;Carudol;Ciperazin;Citrazine;Coopane;Dak;Demovermil;Diatesurico;Dicevermin;Digesan;Dilaurazine;Dispermin;Diurazina;Dowzene;Ecosan;Endorid;Entazin;Equizole-a;Escovermin;Esteropipate;Etaphylline (acetyllinate);Gentiazina;Glycopiparsol;Heksapar;Helmacid;Helmezin;Helmicide;Helmifren;Helmipar;Helmirazine (adipate);Helmirazine (citrate);Helmitin;Helmizin;Herb royal round worm treatment;Hexanthelin;Ismiverm;Janes liquid permifu;Jarabe neox;Jetsan supp. (adipate);Justalmin;Kennel-maid;Kihomato;Kontipar;Lamboxil;Lombricida tropico;Lombrifher;Lombrikal;Lombrimade;Mapiprin;Maskito;Noxiurotan;Ogen;Okuside;Optiverm;Oxiril syrup (hydrate);Oxiuran (hydrate);Oxiurasin;Oxiustip elix;Oxivermin;Oxizin;Oxucid;Oxuril;Oxypip;Oxyzin;P.c. (citrate);Padrax;Paravermin;Pariamate;Par-tega;Perin;Piavermit;Pincide;Pipan;Pip-a-ray;Pipenin;Piperacid;Piperamicin;Piperascat;Piperaskat;Piperate;Piperaverm;Piperazinal;Piperazine (adipate);Pipercrean;Piperex;Piperiod;Piperital od;Piperitol;Piper-jodina;Piperol fort;Piperone;Piperoverm;Pipertox;Piperver;Piperzinal;Pipeverm;Pipezol;Pipizan citrate;Pipracid;Piprazid;Piprazyl;Pipricide;Piptelate;Piverma;Polo-verm;Polyquil;Pripsen;Provtovermil;Razinol;Rondelim;Rondoxyl;Santoban;Siropar;Supraverm;Taenifigin;Teniver;Tivazine;Toxocan;Uricida;Uridina;Uroclear (hexamine);Urodan (phosphate);Urosolvina;Uvilon syrup (hydrate);Vanpar (hydrate);Veripar;Vermazine;Vermenter;Vermicompren;Vermidol;Vermifug;Vermilass;Vermipan;Vermiphsarmette;Vermiquimpe;Vermiquimyc;Vermisit;Vermitox;Vermofrik;Verocid;Wairmex;Wurmex;Wurmsirup siegfried Multifuge;Multifuj;Nea-vermiol;Nemafugan;Nemasin;Nematocton;Nematorazine;Neo-ifusa;.
- World Health Organization (WHO)
- Piperazine was first used as a treatment for gout earlier this century and its anthelminthic activity was discovered in 1949. It is also considerably cheaper than other anthelminthic drugs. In some countries where ascariasis is not endemic and where piperazine was used predominantly for the treatment of pinworm it has been withdrawn from use on the grounds that other more effective and less toxic drugs are now available (see full list). In other such countries, however, piperazine remains available in over-the-counter preparations. Clinical dosages occasionally induce transient neurological signs and concern has been expressed that in some circumstances the drug may generate small amounts of nitrosamine in the stomach. However, it is widely considered that these trace doses are unlikely to give rise to a significant carcinogenic potential. (Reference: (WHODIB) WHO Drug Information Bulletin, 1: 5, , 1983)
- General Description
- Needle-like white or colorless crystals. Shipped as a solid or suspended in a liquid medium. Very corrosive to skin, eyes and mucous membranes. Solid turns dark when exposed to light. Flash point 190°F. Used as a corrosion inhibitor and as an insecticide.
- Air & Water Reactions
- Flammable. Absorbs water and carbon dioxide from air. Soluble in water.
- Reactivity Profile
- 1,4-Diazacyclohexane neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Absorbs carbon dioxide from the air, which can cause dry crystals to seem to melt. May generate hydrogen, a flammable gas, in combination with strong reducing agents such as hydrides. 1,4-Diazacyclohexane is sensitive to light; 1,4-Diazacyclohexane absorbs water and carbon dioxide from air. 1,4-Diazacyclohexane may be corrosive to aluminum, magnesium and zinc. .
- Health Hazard
- TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
- Health Hazard
- Piperazine is a corrosive substance. The solidand its concentrated aqueous solutions areirritants to the skin and eyes. The irritanteffect in rabbits’ eyes was severe.
The toxic symptoms from ingestion ofpiperazine include nausea, vomiting, excitement,change in motor activity, somnolence,and muscle contraction. The toxicity of thiscompound is low, however. The oral LD50value in rats is 1900 mg/kg. The inhalationtoxicity is very low. The inhalation LC50value in mice is 5400 mg/m3/2 h.
- Fire Hazard
- Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
- Pharmaceutical Applications
- A synthetic chemical, most commonly formulated as the citrate, but also available as the adipate, edetate calcium and tartrate salts.
- Contact allergens
- Piperazine is contained in pyrazinobutazone, an equimolar salt of piperazine and phenylbutazone. Among occupational cases, most were reported in the pharmaceutical industry or laboratory workers, in nurses, and in veterinarians.
- Pharmacokinetics
- Activity against intestinal worms requires that a substantial amount remains in the gut. However, after oral administration a variable amount is rapidly absorbed from the small intestine and subsequently excreted in the urine. Its half-life is extremely variable.
- Clinical Use
- Hexahydropyrazine or diethylenediamine (Arthriticine,Dispermin) occurs as colorless, volatile crystals of the hexahydratethat are freely soluble in water. After the discoveryof the anthelmintic properties of a derivative diethylcarbamazine,the activity of piperazine itself was established.Piperazine is still used as an anthelmintic for the treatmentof pinworm (Enterobius [Oxyuris] vermicularis) and roundworm(Ascaris lumbricoides) infestations. It is available invarious salt forms, including the citrate (official in the USP)in syrup and tablet forms. Piperazine blocks the response of the ascaris muscleto acetylcholine, causing flaccid paralysis in the worm,which is dislodged from the intestinal wall and expelled inthe feces.
- Clinical Use
- Ascariasis
Pinworm
- Side effects
- Some people develop hypersensitivity, requiring cessation of treatment. Transient, mild gastrointestinal or neurological symptoms may occur.
- Safety Profile
- Moderately toxic by ingestion, skin contact, intravenous, and subcutaneous routes. Mildly toxic by inhalation. A skin and severe eye irritant. Excessive absorption can cause urticaria, vomiting, diarrhea, blurred vision, and weakness. Combustible when exposed to heat or flame; can react vigorously with oxidizing materials. Explodes on contact with dicyanofurazan. To fight fire, use alcohol foam, mist, dry chemical, water spray. When heated to decomposition it emits highly toxic fumes of NOx.
- Chemical Synthesis
- Piperazine (38.1.12) is a bulk product in organic synthesis. It is made from ethanolamine by heating it in ammonia at a temperature of 150–220°C and a pressure of 100–250atm. It is used as a drug in the form of a salt, and as a rule, in the form of adipinate.
![]()
- Potential Exposure
- (Piperazine): Primary irritant (w/o allergic reaction),
- Veterinary Drugs and Treatments
- Piperazine is used for the treatment of ascarids in dogs, cats, horses, swine and poultry. Piperazine is considered safe to use in animals with concurrent gastroenteritis and during pregnancy.
- Carcinogenicity
- No increase in lung adenomas was produced in mice administered 0.69–18.75mg of piperazine/ kg in drinking water for 20–25 weeks and sacrificed 10–13 weeks later. Mice fed the equivalent of 938 mg/kg in the diet for 28 weeks and sacrificed at 40 weeks failed to show any significant increase in the incidence of lung adenomas. An increase in lung adenomas was produced in this bioassay by administration of piperazine together with sodium nitrate, suggesting the formation of the active nitroso derivative. Sodium ascorbate inhibited tumor formation, in theory, by preventing piperazine nitrosation (304). Coadministration of 250 ppm piperazine and 500 ppm sodium nitrate in drinking water did not produce tumors in rats. None of these studies were conducted using currently accepted methods for evaluating carcinogenic potential but piperazine alone, in these assays, was noncarcinogenic.
- Environmental Fate
- This molecule has a simple chemical structure and molecular weight of 86.14. It has a strong alkaline base soluble in water (1:18), glycerol, and glycols, but is only sparingly soluble in alcohol and insoluble in ether. Piperazine is not expected to hydrolyze in water. The photodegradation half-life is approximately 0.8 h. The piperazine molecule is easily denaturalized by diverse environmental factors and has a low potential for bioaccumulation or biomagnification. To improve its stability, it is usually formulated as different salts such as adipate, citrate, phosphate, hexahydrate, and sulfate. Most piperazine salts are white crystalline powders that are readily soluble in water.Exceptions are adipates, which dissolve to only a maximum concentration of 5% in water, and phosphate, which is insoluble.
- Shipping
- UN2579 Piperazine, Hazard class: 8; Labels: 8-Corrosive material.
- Purification Methods
- Piperazine crystallises from EtOH or anhydrous *benzene and is dried at 0.01mm. It can be sublimed under vacuum and purified by zone melting. The hydrochloride has m 172-174o (from EtOH), and the dihydrochloride crystallises from aqueous EtOH and has m 318-320o (dec, sublimes at 295-315o). The picrate has m ~200o, and the picrolonate crystallises from dimethylformamide ( m 259-261o). [Beilstein 23 H 4, 23 I 4, 23 II 3, 23 III/IV 15, 23/1 V 30.]
- Toxicity evaluation
- Piperazine blocks transmission by hyperpolarizing nerve membranes at the neuromuscular junction, leading to parasite immobilization by flaccid paralysis and consequent removal from predilection and death. Piperazine is a selective agonist of GABA receptors, resulting in the opening of chloride channels and hyperpolarization of the membrane of the muscle cells of nematode parasites.
- Incompatibilities
- Aqueous solution is a strong base. Violent reaction with strong oxidizers and dicyanofurazan. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitrogen compounds, carbon tetrachloride. Attacks aluminum, copper, nickel, magnesium and zinc.
-
Tel:
Update Time:2024/11/15
-
Tel:
Update Time:2024/11/15
-
Tel:
Update Time:2024/06/17
-
Tel:
Update Time:2024/11/02
-
Tel:
Update Time:2024/05/22
10. Related Questions
- What are the uses of Piperazine?The term pharmaceutical intermediate refers to the components that have good pharmaceutical properties and can achieve the desired effects. Therefore, when discussing various chemical components, peop..
- What is Piperazine and How is it Used? Piperazine is an organic compound with a unique six-membered ring structure containing two nitrogen atoms. Originally used as a solvent for uric acid, it was later discovered to have anthelmintic pro..
- Number of total stereoisomers of a piperazine derivative How do I find out the total number of stereoisomers of 3,6-dimethylpiperazine-2,5-dione? As far as I can see, the compound has two chiral carbons. Would the nitrogens make a difference? They seem chi..
- Stable conformers of piperazine which conformer stable of piperazine, when hydrogen of N in axial or in equatorial, or lone pair? why?
11. Realated Product Infomation

 EN
EN

 C
C