-
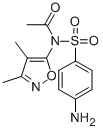
SULFISOXAZOLE ACETYL (200 MG)
- CAS:80-74-0
- MW:309.3409
- MF:C13H15N3O4S
Sulfisoxazole acetyl shares the actions and usesof the parent compound, sulfisoxazole. The acetyl derivativeis tasteless and, therefore, suitable for oral administration, especiallyin liquid preparations. The acetyl compound is splitin the intestinal tract and absorbed as sulfisoxazole; that is, itis a prodrug for sulfisoxazole. SULFISOXAZOLE ACETYL (200 MG) Preparation Products And Raw materials Raw materials
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. MSDS
5. NMR Spectrum
6. Synthesis Route

 EN
EN