-

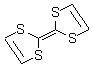
TETRATHIAFULVALENE
- CAS:31366-25-3
- MW:204.35600
- MF:C6H4S4
Electron donor for supramolecular synthesis,1 charge-transfer complex synthesis2 with 7,7,8,8-tetracyanoquinodimethane (cat. no. 157635), and for electron transfer to diazonium salts.3
View more+
 EN
EN