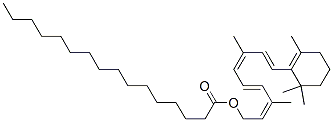
Retinyl palmitate is an ester of Retinol and is the major form of vitamin A found in the epidermis. Retinyl palmitate has been widely used in pharmaceutical and cosmetic formulations.
Solid
All-trans-retinyl palmitate is an all-trans-retinyl ester obtained by formal condensation of the carboxy group of palmitic (hexadecanoic acid) with the hydroxy group of all-trans-retinol. It is used in cosmetic products to treat various skin disorders such as acne, skin aging, wrinkles, dark spots, and also protect against psoriasis. It has a role as an Escherichia coli metabolite, a human xenobiotic metabolite and an antioxidant. It is a retinyl palmitate and an all-trans-retinyl ester. It derives from an all-trans-retinol.|Retinyl Palmitate is a naturally-occurring phenyl analogue of retinol (vitamin A) with potential antineoplastic and chemopreventive activities. As the most common form of vitamin A taken for dietary supplementation, retinyl palmitate binds to and activates retinoid receptors, thereby inducing cell differentiation and decreasing cell proliferation. This agent also inhibits carcinogen-induced neoplastic transformation, induces apoptosis in some cancer cell types, and exhibits immunomodulatory properties. (NCI04)

 EN
EN

 T,?
T,? Xn
Xn