Phosphoryl bromide reaction with benzoic acid
Earle
Answered Nov 11 2021
I am trying to find out how POBrX3 reacts with ?COOH group (benzoic acid to be specific). I found that it performs an elimination reaction with alcohols to give alkenes. I have also seen that keto group was converted to ?Cl using POClX3.
But I don't think that POClX3 will eliminate ?COOH group — that seems like nonsense. Eliminating using ?OH from ?COOH would give me a carbon with five bonds. Exchanging the =O from ?COOH would give me something I have never seen and it doesn't even look good…
So I am sort of out of ideas. I'd appreciate any hint what to do.
Daley
Answered Nov 11 2021
So the correct answer (according to my teacher) is that R-COBr is generated from the reaction of POBr3 with R-COOH (benzoic acid)
Edmund
Answered Nov 11 2021
Well actually @Waylander and @Nilay Ghosh are both correct in a way. To convert carboxylic acids into acyl chloride, PClX5 is often used as a chlorinating agent , which yields an acyl chloride and POClX3 (and HCl) as a by-product.
This POBrX3 (or likewise POClX3) is essentially a dehydrating agent and is thus used to dehydrate alcohols to alkenes(1)
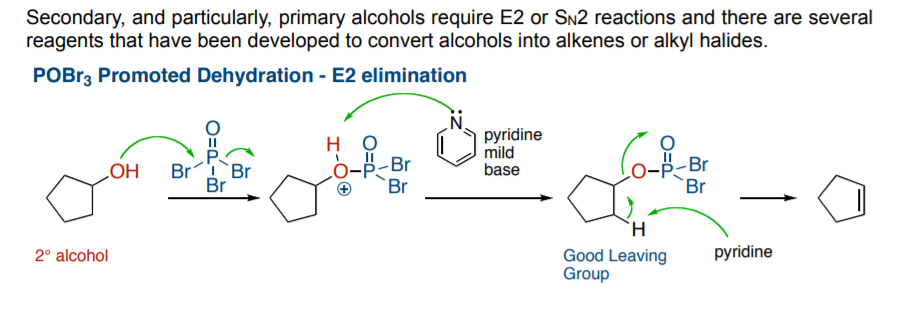
or brominate(2) (in some cases) to form alkyl bromides.
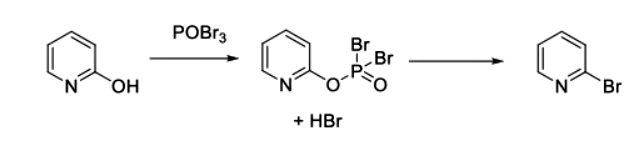
But this dehydrating capability might also be used on carboxylic acids to convert them into their anhydrides just like PX4OX10, though there's no such special mention of the viability of the reaction progress.
But since you're really asking, I think conversion of the acid into its anhydride could be a possible outcome in presence of a dehydrating agent POBrX3.







