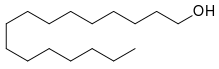
This is simply how the chemist chose to draw the molecule in that particular instance, and cetyl alcohol is formally regarded as a straight-chain molecule. Cetyl alcohol has all single bonds and no rings, so it is not structurally locked into any particular conformation or geometry, and every bond is free to rotate.
For very simple saturated molecules like this, where the conformation is not in any way fixed (under typical conditions), the chemist is free to represent the molecule as one of any of its possible conformers.
In reality, the most stable (i.e., lowest energy) conformer should statistically predominate, and this is the one with all groups staggered and the largest groups antiperiplanar (q.v., the IUPAC Gold Book entry on torsion angles for details), the conventional explanation being that this minimizes steric hindrance/strain. The skeletal formula corresponding to this minimum-energy conformer is the simple straight-chain representation (seen here, for example).