What are the properties and production method of 4,4'-Diamino-2,2'-stilbenedisulfonic acid (DABS)?
Background and Overview
4,4'-Diamino-2,2'-stilbenedisulfonic acid, also known as DABS, is primarily used in the production of fluorescent whitening agents, Direct Yellow G, Direct Yellow R, Sunfast Orange F3G, and insect repellents. It is toxic and can cause skin irritation. Avoid ingestion and direct contact with the body. Store in a cool, ventilated warehouse, and keep it sealed. The storage period is six months. Handle and transport according to regulations for toxic chemicals.
Physical and Chemical Properties
4,4'-Diamino-2,2'-stilbenedisulfonic acid, or DABS, is an organic compound. Here are some properties of DABS:
1. Appearance: DABS is a white to pale yellow crystalline powder.
2. Solubility: It is almost insoluble in water but can dissolve in some organic solvents like alcohols and ethers.
3. Acidity: DABS is an acidic compound that can react with bases to form salts.
4. Stability: It is relatively stable at room temperature but may decompose at high temperatures or when in contact with strong oxidizing agents.
Production Method
4,4'-Diamino-2,2'-stilbenedisulfonic acid is a synthetic organic compound. The synthesis reaction is shown in the image below:
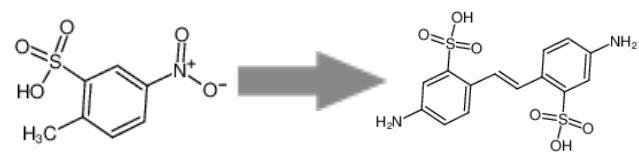
Figure 1: Synthesis reaction of 4,4'-Diamino-2,2'-stilbenedisulfonic acid
The production process involves the following steps:
1. Prepare styrene and sulfuric acid waste. Styrene is a common chemical raw material that can be extracted from petroleum. Sulfuric acid waste is obtained through acid-base neutralization reactions.
2. Add styrene to a reaction vessel and heat it to the appropriate temperature. Slowly add sulfuric acid waste to the reaction vessel while stirring.
3. Control the reaction temperature and time to promote the reaction. The reaction temperature is typically maintained between 80-100 degrees Celsius.
4. After the reaction, cool the reaction mixture and add enough acidic solution for neutralization.
5. The precipitate formed after neutralization can be separated and collected through centrifugation or filtration.
6. The collected precipitate is dried to obtain the final product of 4,4'-Diamino-2,2'-stilbenedisulfonic acid.
If large-scale production is required, process optimization and economic considerations need to be taken into account.
References
[1] Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 1, p. 510




