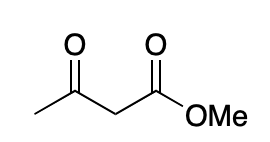
The enol forms of beta-diketones and beta -ketoesters are stabilized
by intramolecular hydrogen bonds in its enolic form. Enol structure with greater conjugation is more stable and amajor enol.
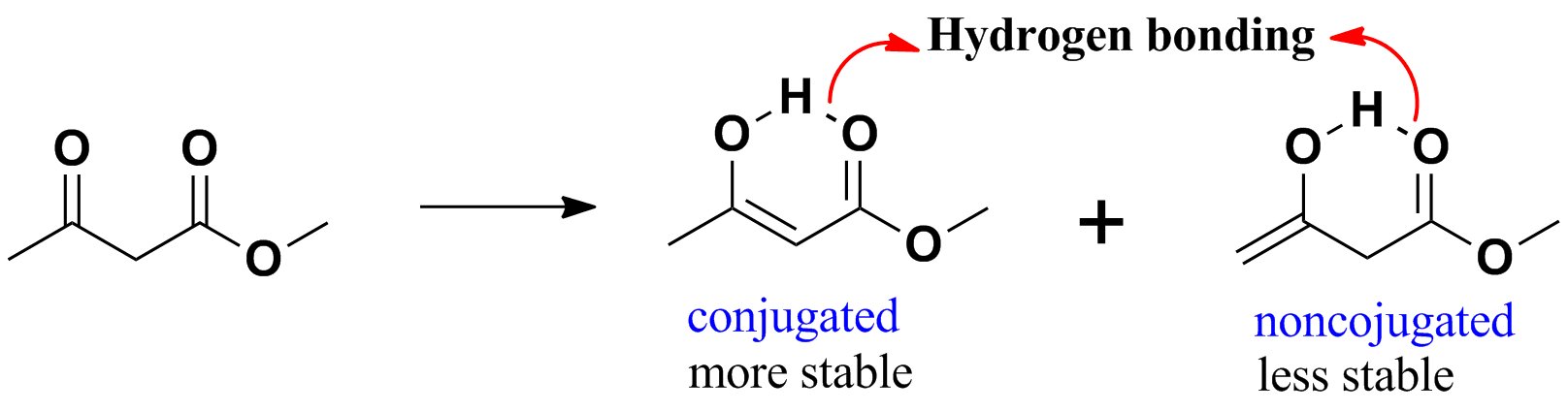
update :
In organic chemistry terms, conjugation is used to describe the situation that occurs when π systems (e.g. double bonds) are "linked together".
A conjugated system requires that there is a continuous array of "p" orbitals that can align to produce a π bonding overlap along the whole system.If a position in the chain does not provide a "p" orbital or if geometry prevents the correct alignment, then the conjugation is interrupted (broken) and therefore lost at that point. Due to extra π bonding interactions between the adjacent π systems there is an overall stabilization of the system .This is referred to as the delocalisation energy or the resonance energy or conjugation energy
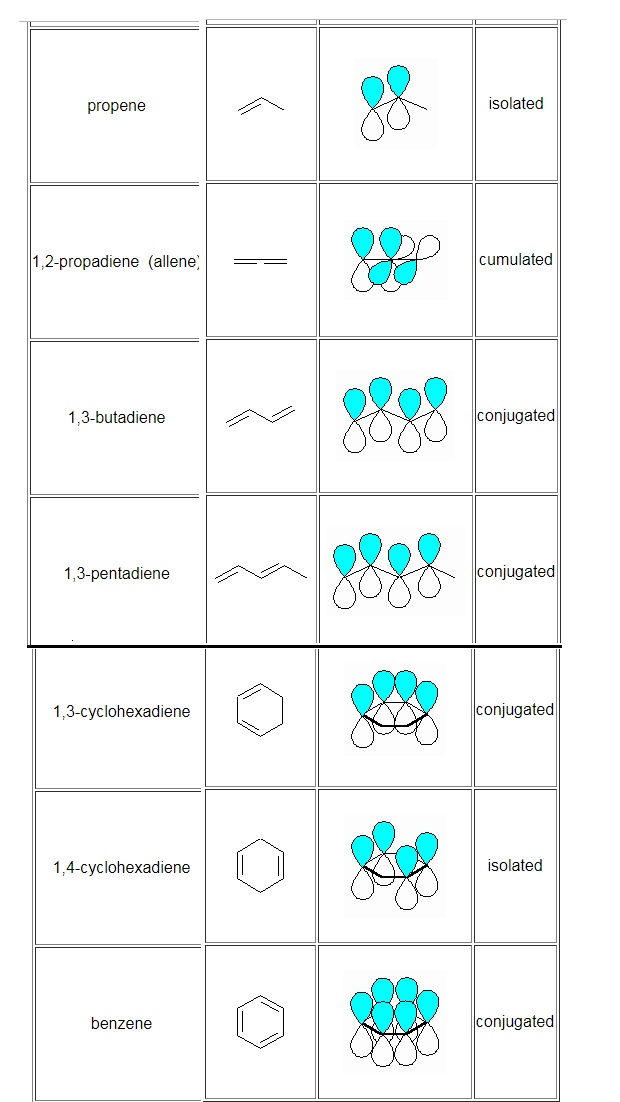
source:
http://www.chem.ucalgary.ca/courses/350/Carey5th/Ch10/ch10-1-1.html
According to MO theory,1,3-butadiene ,comprised of four overlapping p orbitals are mathematically combined to produce four molecular orbitals.As seen in figure , the four π electrons of a conjugated diene occupy the two lowest energy MOs (the bonding MOs).Specifically, the two electrons that occupy ψ1 are delocalized over four carbon atoms. This delocalization accounts for the observed stabilization energy .
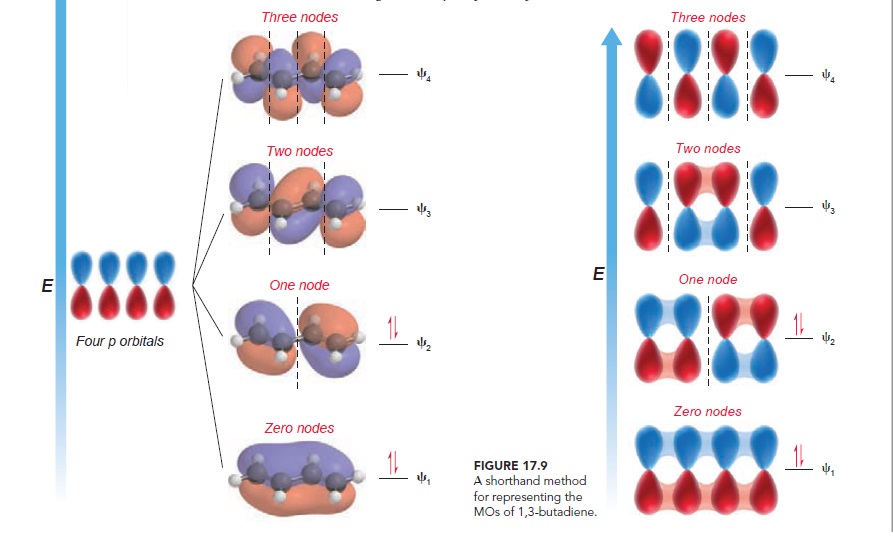 source :
Organic chemistry ,David R. Klein.page no.773
source :
Organic chemistry ,David R. Klein.page no.773
ref :Advanced Organic ,Chemistry FIFTH EDITION ,Part A: Structure and Mechanisms FRANCIS A. CAREY
and RICHARD J. SUNDBERG