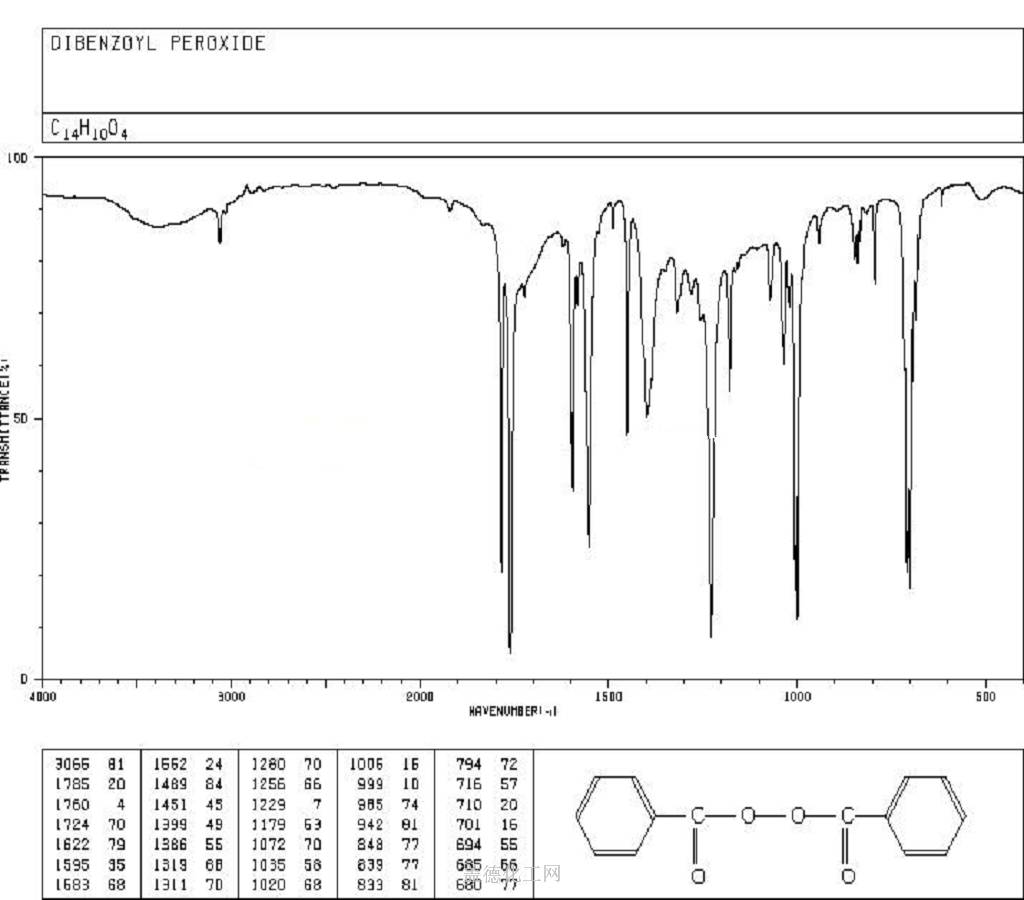
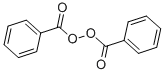
-
Benzoyl peroxide
- CAS:94-36-0
- MW:242.2268
- MF:C14H10O4
Benzoyl peroxide, with the chemical formula C14H10O4 and CAS registry number 94-36-0, is a widely used organic compound known for its effectiveness as a medication for acne treatment. This white, granular solid is characterized by its peroxide group attached to the benzoyl group. It functions by releasing oxygen free radicals, which oxidize comedones (clogged pores), thus reducing inflammation and killing bacteria on the skin surface. Benzoyl peroxide is available in various forms, including creams, gels, and washes, and is often used in combination with other acne treatments. It is considered a first-line treatment for mild to moderate acne and is available over-the-counter in many countries. Additionally, it has applications in polymerization reactions, as a bleaching agent, and in the synthesis of other organic compounds. Benzoyl Peroxide may affect workers in the electronics and plastics (epoxy resins and catalysts) industries, electricians,ceramic workers, dentists and dental technicians,laboratory technicians and bakers. As it wascontained in candles, it also induced contact dermatitisin a sacristan.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. Safety and Handling
5. MSDS
6. NMR Spectrum
7. Synthesis Route
8. Precursor and Product
9. Computed Properties
12. Related Questions
13. Realated Product Infomation

 EN
EN