-
COBALT(II) OXALATE DIHYDRATE
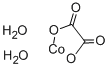
COBALT(II) OXALATE DIHYDRATE, with the chemical formula C4H2CoO6·2H2O and CAS registry number 5965-38-8, is a compound known for its unique properties and applications. This compound is a coordination complex consisting of cobalt(II) ions, oxalate ions, and water molecules. It is a green crystalline solid that is soluble in water. COBALT(II) OXALATE DIHYDRATE is commonly used as a catalyst in various chemical reactions, particularly in organic synthesis. It can also be used as a precursor for the preparation of other cobalt compounds. Additionally, this compound has been studied for its potential applications in electrochemical devices and as a luminescent material. Overall, COBALT(II) OXALATE DIHYDRATE is a versatile compound with diverse uses in the field of chemistry.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. MSDS
5. NMR Spectrum
8. Realated Product Infomation

 EN
EN