Ethyl alcohol, also called ethanol, absolute alcohol, or grain alcohol, is a clear, colorless, flammableliquid with a pleasant odor. It is associated primarily with alcoholic beverages, but ithas numerous uses in the chemical industry. The word alcohol is derived from the Arabicword al kuhul, which was a fine powder of the element antimony used as a cosmetic. InMedieval times, the word al kuhul came to be associated with the distilled products knownas alcohols. The hydroxyl group, -OH, bonded to a carbon, characterizes alcohols. Ethyl isderived from the root of the two-carbon hydrocarbon ethane.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. Safety and Handling
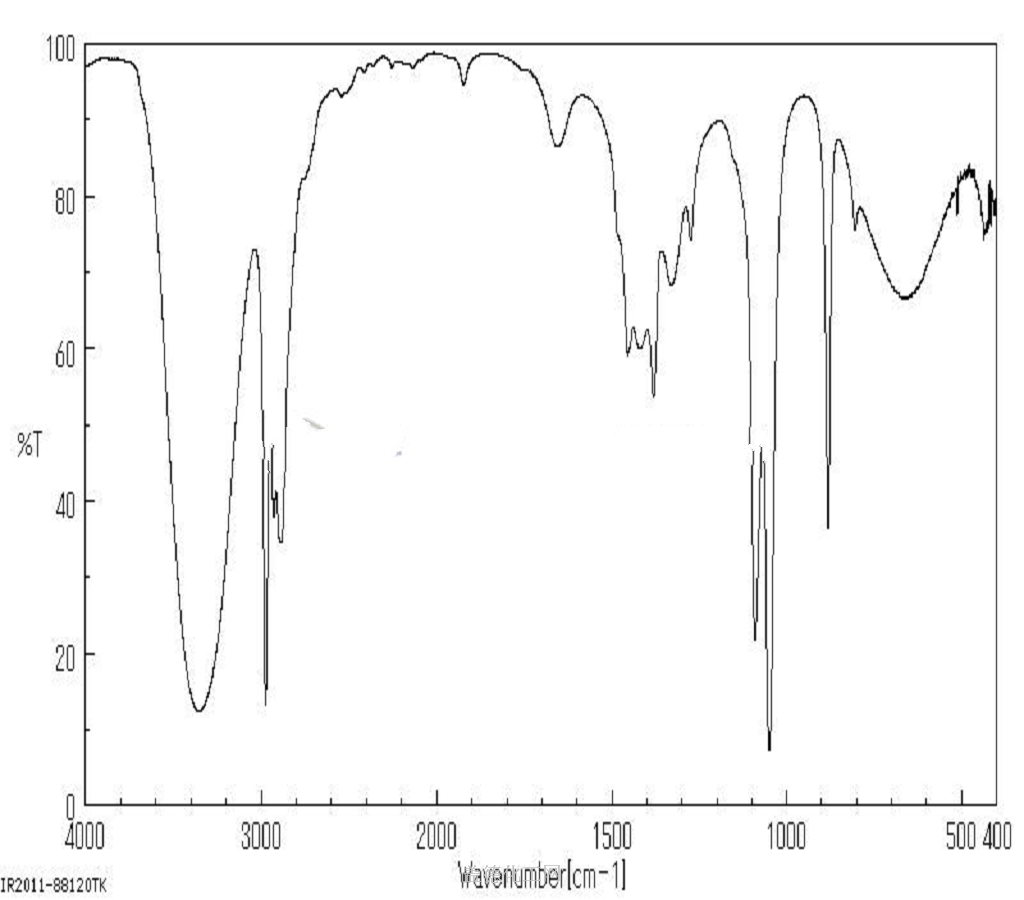
5. MSDS
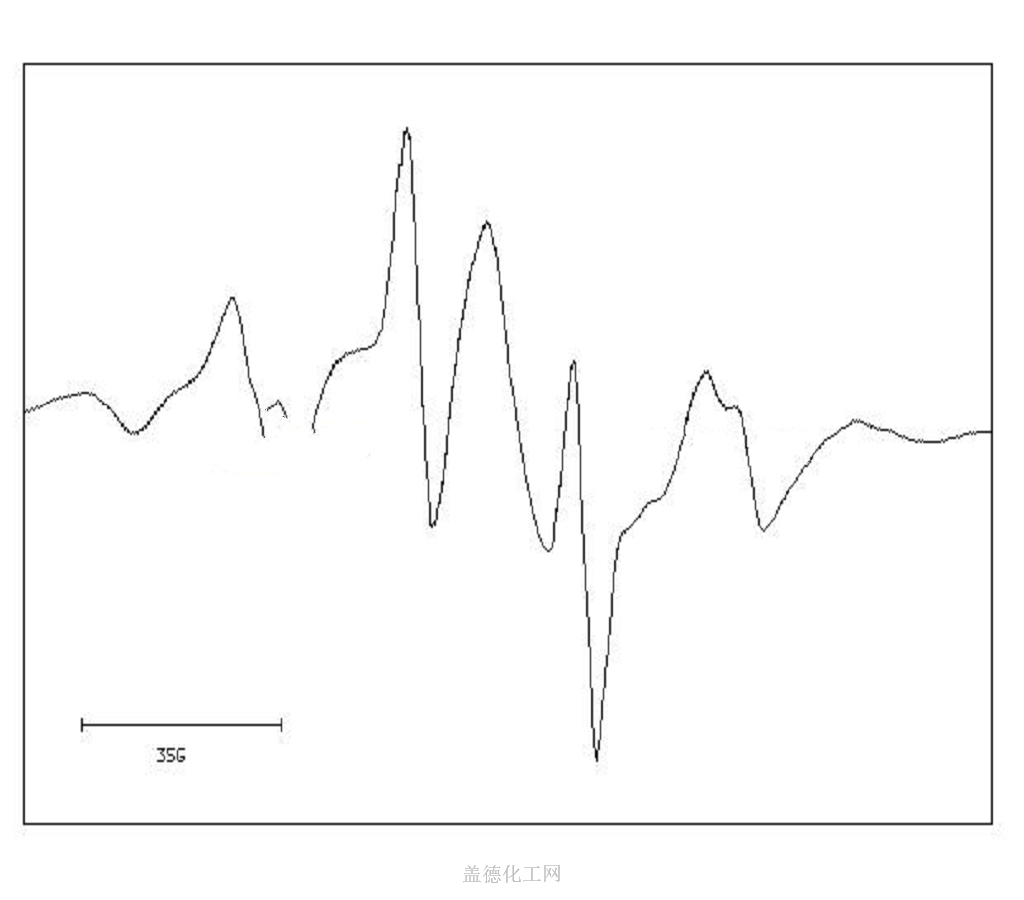
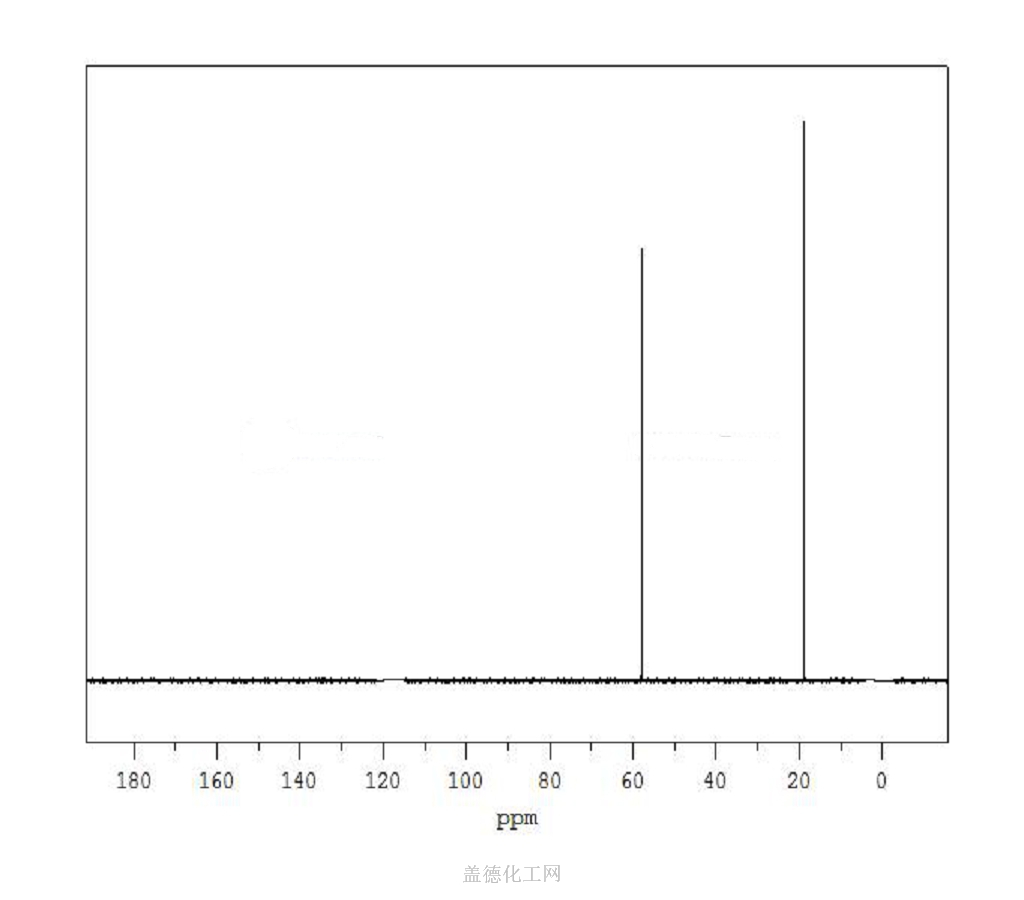
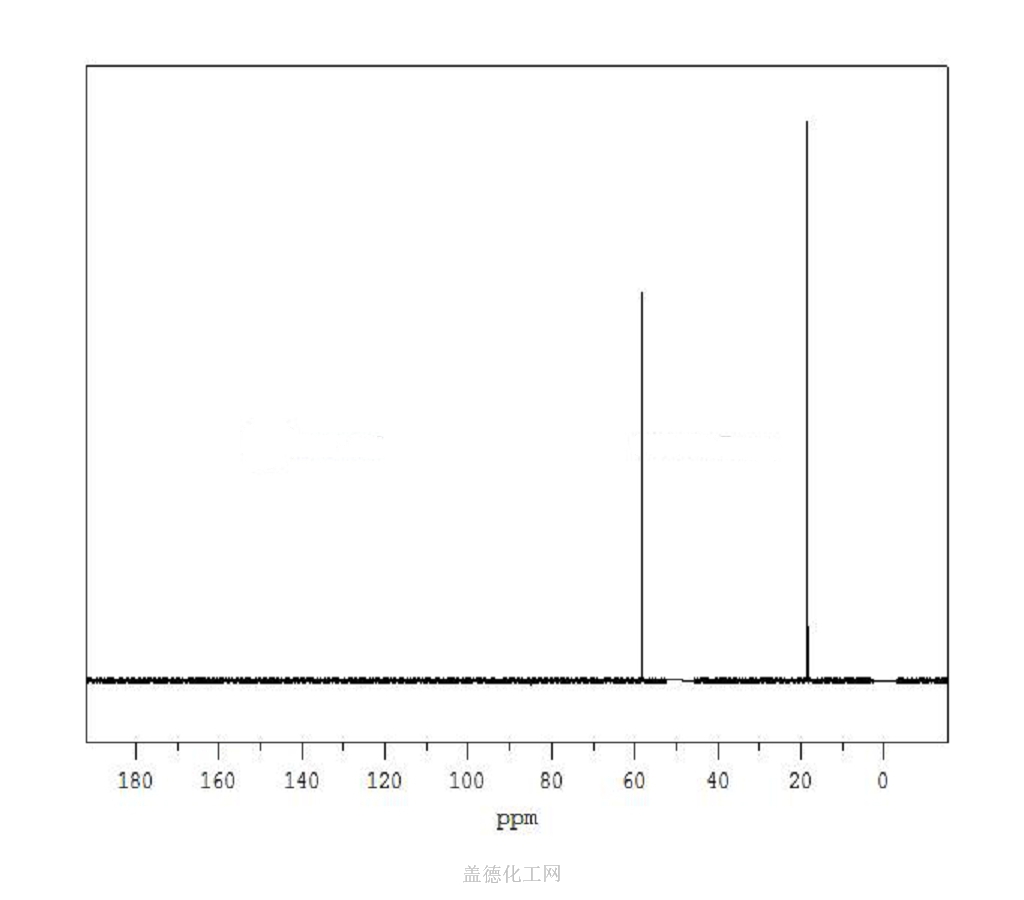
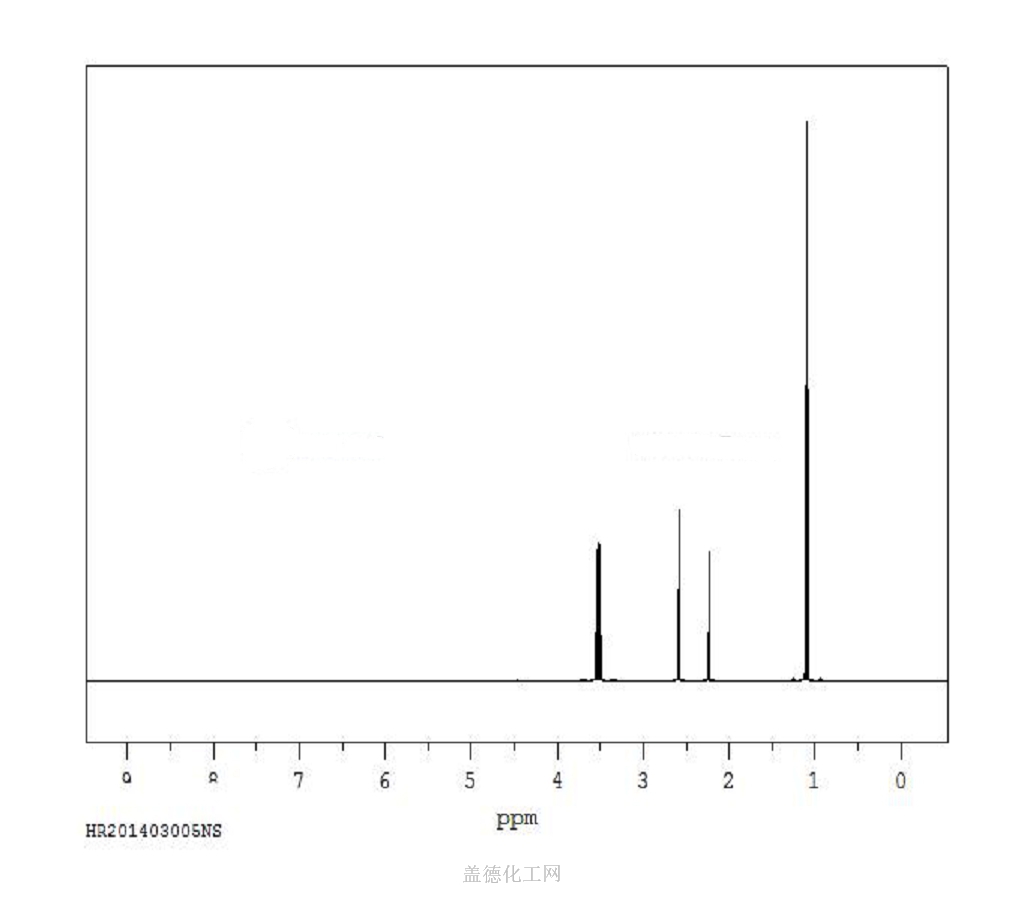
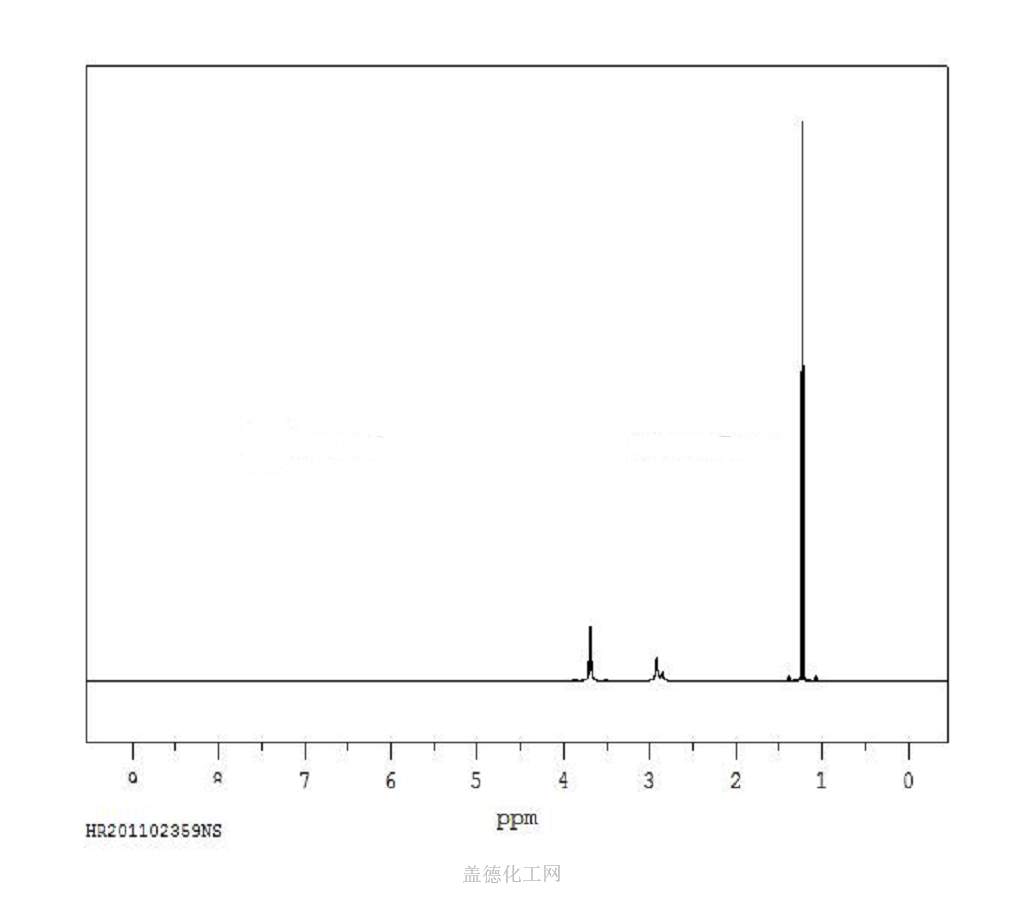
6. NMR Spectrum
7. Synthesis Route
8. Precursor and Product
9. Computed Properties
12. Related Questions
13. Realated Product Infomation

 EN
EN