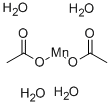
Manganese(II) Acetate Tetrahydrate is a chemical compound with the CAS number 6156-78-1. It appears as a pink crystalline solid with a slight acetic acid odor. The molecular formula of Manganese(II) Acetate Tetrahydrate is Mn(CH3COO)2 · 4H2O. It is soluble in water and forms a clear pink solution.
Applicable Fields
Manganese(II) Acetate Tetrahydrate is commonly used in various fields for different purposes. In the field of chemistry, it is used as a catalyst in organic synthesis reactions. It can also be used as a reagent in laboratory experiments. In the field of agriculture, it is used as a micronutrient fertilizer to provide plants with essential manganese. Manganese(II) Acetate Tetrahydrate is also used in the production of pigments, dyes, and ceramics.
Mechanism of Action
In organic synthesis reactions, Manganese(II) Acetate Tetrahydrate acts as a catalyst, facilitating the conversion of reactants into desired products. It helps in speeding up the reaction and increasing the yield of the desired product. As a micronutrient fertilizer, it provides plants with manganese, which is essential for various physiological processes, including photosynthesis and enzyme activation. In the production of pigments, dyes, and ceramics, Manganese(II) Acetate Tetrahydrate imparts color and enhances the properties of the final product.
Safety Information
Manganese(II) Acetate Tetrahydrate should be handled with care. It may cause irritation to the skin, eyes, and respiratory system. Prolonged or repeated exposure to this compound may cause adverse health effects. It is important to use appropriate protective measures, such as gloves and goggles, when handling Manganese(II) Acetate Tetrahydrate. In case of ingestion or inhalation, seek medical attention immediately.
Storage
Conditions: Store in a cool and dry place, away from direct sunlight.

 EN
EN