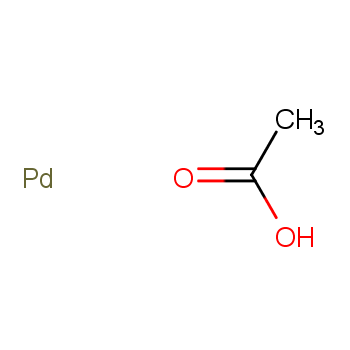
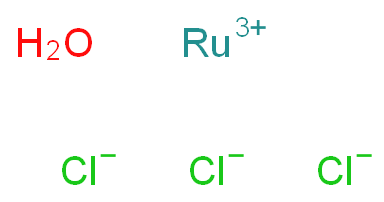Catalysts are essential substances that expedite chemical reactions by reducing the energy needed for activation. They hold paramount significance in industries and biological systems alike. An intrinsic trait of catalysts is their endurance—they remain unaltered throughout reactions, enabling repeated use and cost-effectiveness.
Furthermore, catalysts are highly selective, meaning they only work with specific reactions. Each catalyst is designed to interact with particular reactants and promote the desired chemical transformation. This selectivity is crucial in controlling the outcome of a reaction and ensuring that the desired products are formed.
It is important to note that not all reactions can be catalyzed by the same catalyst. Different reactions require different catalysts with specific properties and functionalities. For example, a catalyst that is effective in promoting a hydrogenation reaction may not be suitable for a dehydrogenation reaction. This specificity arises from the unique molecular interactions between the catalyst and the reactants, which determine the reaction pathway and rate.
In some cases, a single reaction may require multiple catalysts to proceed efficiently. These catalysts can work in tandem, each contributing to a specific step in the reaction mechanism. This cooperative action of multiple catalysts allows for complex reactions to occur under mild coAnditions and with high efficiency.
Catalysts Categories
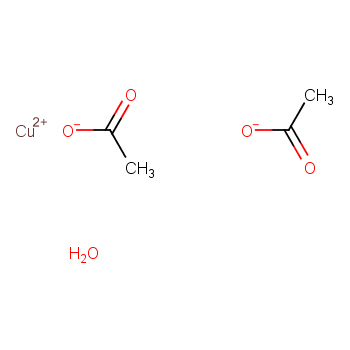
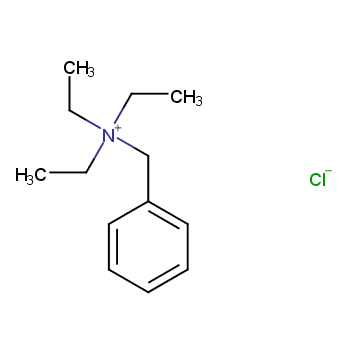
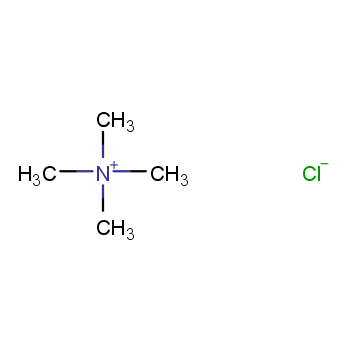
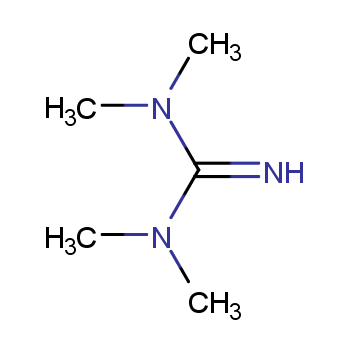
Catalysts encompass diverse categories. They can be classified into liquid and solid forms based on their physical state. Additionally, catalysts are categorized as either homogeneous or heterogeneous, determined by the reaction system's phase.
Homogeneous catalysts comprise acids, alkalis, soluble transition metal compounds, and peroxide catalysts. In contrast, heterogeneous catalysts include solid acid catalysts, organic base catalysts, metal catalysts, molecular sieve catalysts, and biocatalysts.
Another criterion of distinction is the scale of action, giving rise to primary catalysts and co-catalysts. Co-catalysts involve the introduction of substances beyond the catalyst, enhancing its catalytic effect in reactions.
Usage of Catalysts products
The utilization of catalyst products is integral to a multitude of applications, exerting a profound influence on chemical production, scientific experimentation, and everyday activities.
Catalysts assume a pivotal role in accelerating reactions, enhancing efficiency, and unlocking pathways that lead to desired outcomes. This indispensable role extends to diverse domains, including the realms of scientific exploration and routine life functions.
Notably, the brewing industry relies on catalysts to expedite fermentation processes, while the pharmaceutical sector harnesses their power to craft complex molecules with precision.
Overall, catalysts are indispensable tools in the field of chemistry and beyond. Enzymes are highly beneficial in industries such as petroleum refining, pharmaceuticals, and environmental remediation. This is because they have the ability to accelerate reactions without being consumed.
.more+