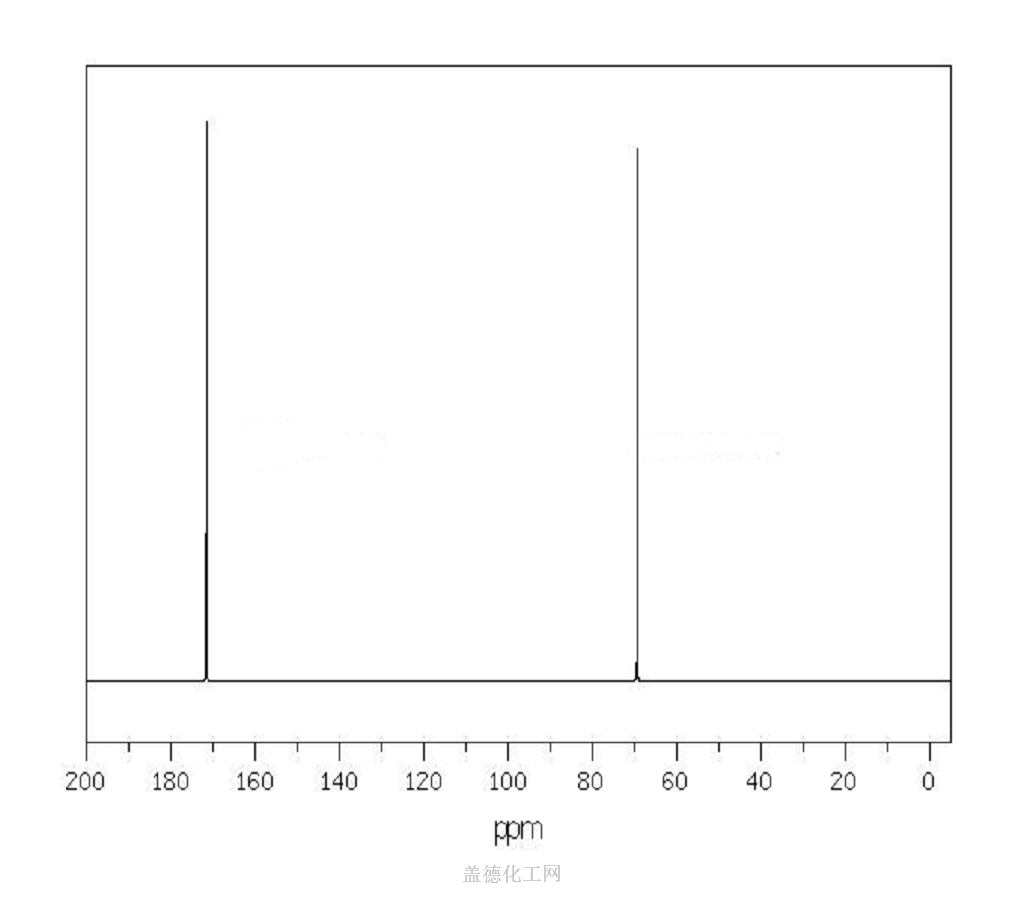
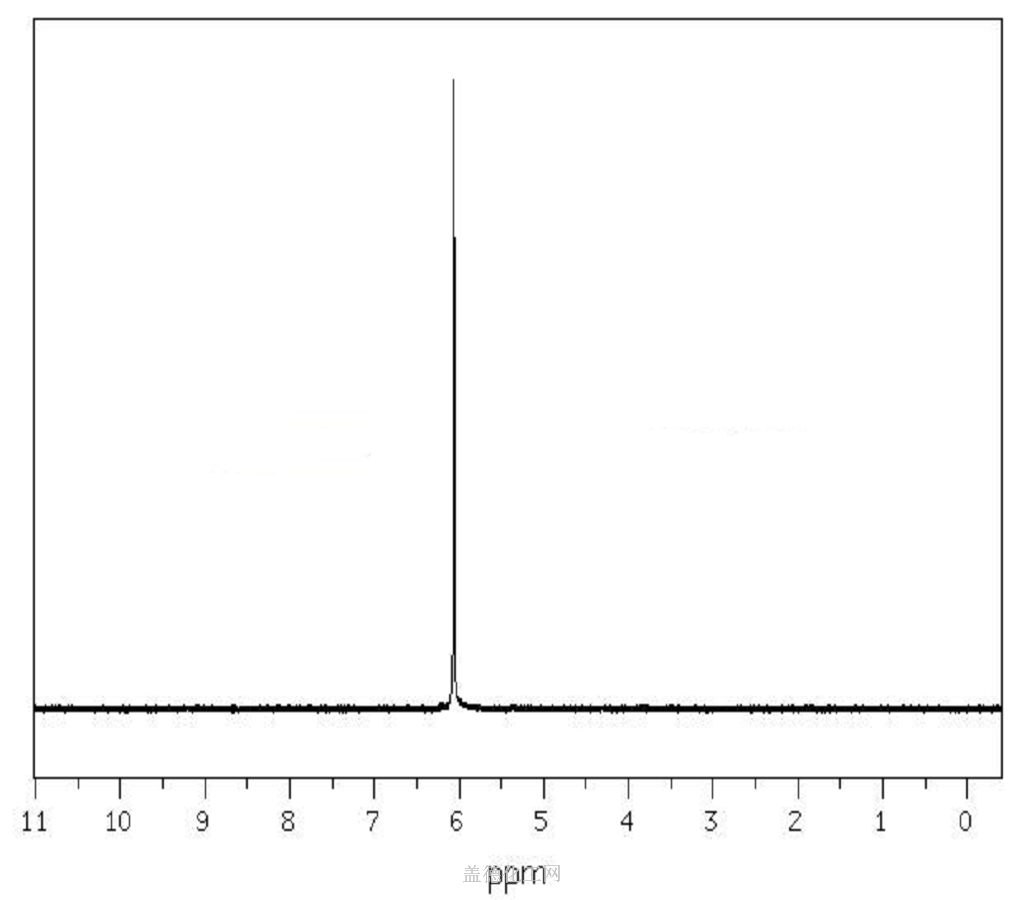
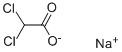The Sodium dichloroacetate, with the cas register number 2156-56-1, has its IUPAC name of sodium 2,2-dichloroacetate. This is a kind of white powder, and is soluble in cold water.
The characteristics of this chemical are as below: (1)H-Bond Acceptor: 2; (2)Rotatable Bond Count: 1; (3)Exact Mass: 149.925129; (4)MonoIsotopic Mass: 149.925129; (5)Topological Polar Surface Area: 40.1; (6)Heavy Atom Count: 7; (7)Formal Charge: 0; (8)Complexity: 64.7; (9)Covalently-Bonded Unit Count: 2.
Uses of Sodium dichloroacetate: Sodium dichloroacetate could react with ethane-1,2-diamine and 4-bromo-phenol to produce dibromo N,N'-ethylenebis[2-(o-hydroxyphenyl)glycines]. The reaction condition is below: reagent: 6 N NaOH; solution:H2O, methanol; reaction time: 24 hours; reaction temp.: 85 ℃; field: 30%.
You should be cautious while dealing with this chemical. Being a kind of irritant chemical, it is irritating to eyes, respiratory system and skin and may cause inflammation to the skin or other mucous membranes. So while using this, you should take the following instructions. Wear suitable protective clothing, gloves and eye/face protection, and if in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
The following datas could be converted into the molecular structure:
(1)Canonical SMILES: C(C(=O)[O-])(Cl)Cl.[Na+]
(2)InChI: InChI=1S/C2H2Cl2O2.Na/c3-1(4)2(5)6;/h1H,(H,5,6);/q;+1/p-1?
(3)InChIKey: LUPNKHXLFSSUGS-UHFFFAOYSA-M?
Below are the toxicity information which have been tested:
| Organism |
Test Type |
Route |
Reported Dose (Normalized Dose) |
Effect |
Source |
| mouse |
LD50 |
intraperitoneal |
> 3gm/kg (3000mg/kg) |
? |
Journal of Agricultural and Food Chemistry. Vol. 10, Pg. 370, 1962. |
| mouse |
LD50 |
oral |
4845mg/kg (4845mg/kg) |
? |
Journal of Pharmacology and Experimental Therapeutics. Vol. 222, Pg. 501, 1982.
? |
| rat |
LD50 |
oral |
5281mg/kg (5281mg/kg) |
? |
Journal of Industrial Hygiene and Toxicology. Vol. 23, Pg. 78, 1941. |

 EN
EN