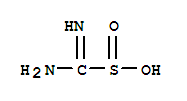THIOUREA DIOXIDE, with the chemical formula CH4N2O2S and CAS registry number 4189-44-0, is a compound known for its versatile applications in various industries. This white crystalline solid, also referred to as Formamidine sulfinic acid, is characterized by its thiourea and dioxide functional groups. It is commonly used as a reducing agent in textile, paper, and leather industries, as well as in photographic processes. THIOUREA DIOXIDE is also utilized in hair dyeing and bleaching products due to its ability to remove unwanted color. Additionally, it finds applications in organic synthesis as a source of sulfur and as a stabilizer for hydrogen peroxide. With its wide range of uses, THIOUREA DIOXIDE plays a crucial role in numerous chemical processes and industries.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. Safety and Handling
5. MSDS
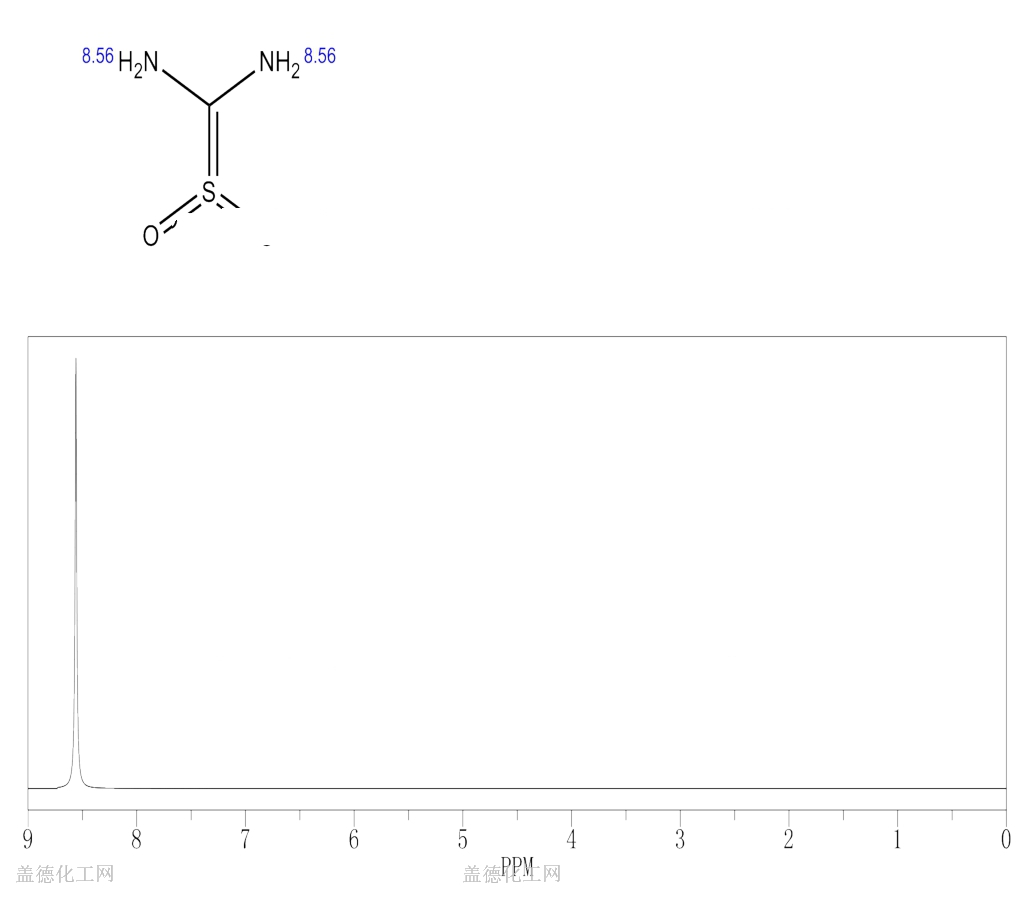
6. NMR Spectrum
7. Synthesis Route

 EN
EN