-

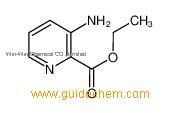
3-Aminopyridine-2-carboxylic acid ethyl ester
- CAS:27507-15-9
- MW:166.1772
- MF:C8H10N2O2
A synthetic intermediate for the synthesis of Kenpaullone derivatives, Cannabinoid receptor ligands, and nociceptive agents. 3-Aminopyridine-2-carboxylic acid ethyl esterSupplier
View more+
 EN
EN