-
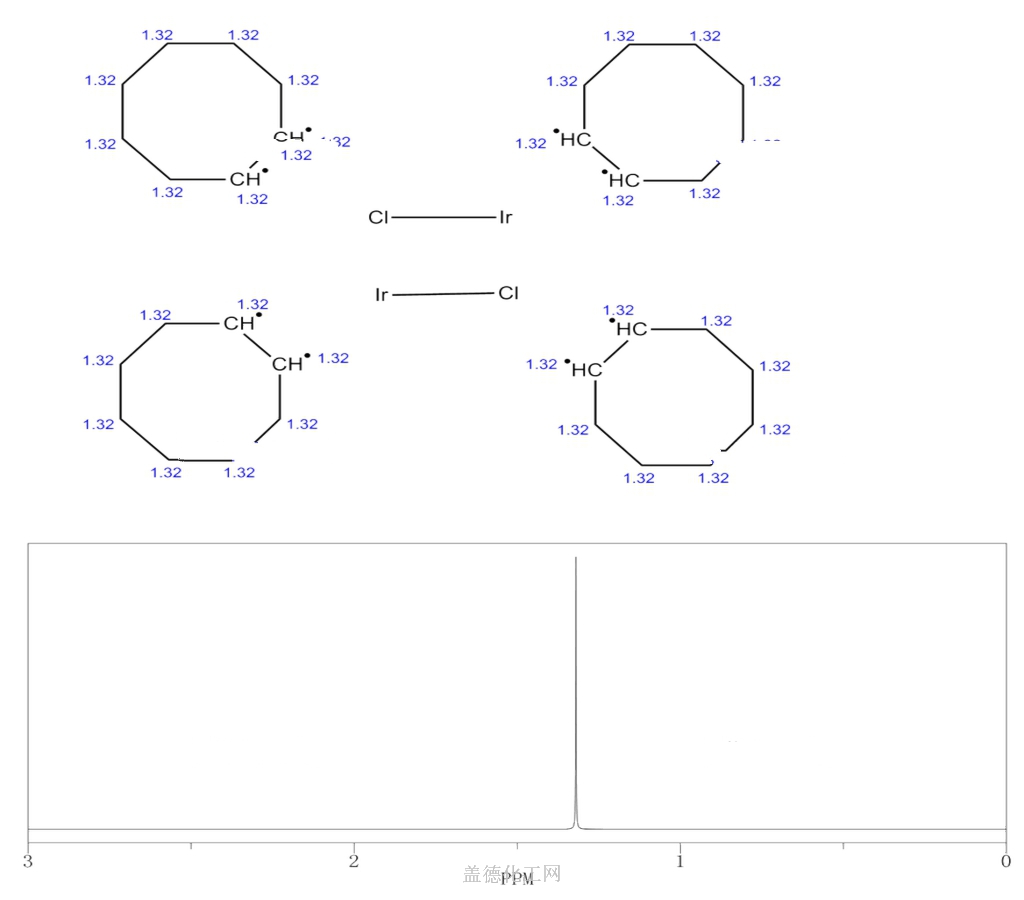
BIS(CYCLOOCTENE)IRIDIUM(I) CHLORIDE, DIMER
BIS(CYCLOOCTENE)IRIDIUM(I) CHLORIDE, DIMER, with the chemical formula C16H28Cl2Ir2 and CAS registry number 12246-51-4, is a compound known for its applications in catalysis and organometallic chemistry. This dimeric complex consists of two cyclooctene ligands coordinated to an iridium(I) center, with chloride ions bridging the two iridium atoms. It is a yellow crystalline solid that is soluble in organic solvents. BIS(CYCLOOCTENE)IRIDIUM(I) CHLORIDE, DIMER is commonly used as a catalyst in various reactions, including hydrogenation, hydroamination, and C-H activation. It exhibits high catalytic activity and selectivity, making it a valuable tool in synthetic chemistry. Additionally, this compound has been studied for its potential applications in the field of photovoltaics, due to its ability to absorb and emit light. Overall, BIS(CYCLOOCTENE)IRIDIUM(I) CHLORIDE, DIMER is a versatile compound with diverse applications in the field of chemistry.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. MSDS
5. NMR Spectrum
6. Computed Properties
9. Realated Product Infomation

 EN
EN