-
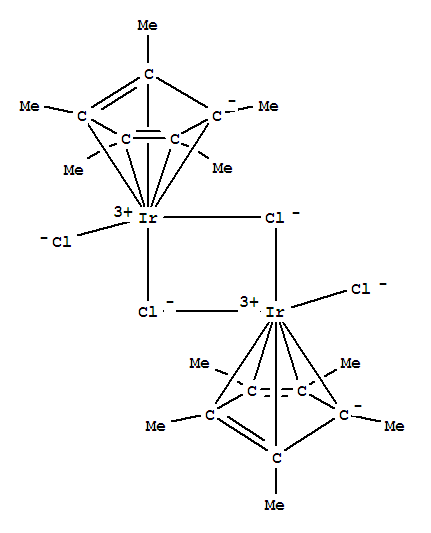
(Pentamethylcyclopentadienyl)iridium(III) chloride dimer
(Pentamethylcyclopentadienyl)iridium(III) chloride dimer, with the chemical formula C15H30Cl2Ir and CAS registry number 12354-84-6, is a compound known for its applications in catalysis and organometallic chemistry. This dark purple solid, also referred to as (Cp*)IrCl2, is characterized by its pentamethylcyclopentadienyl ligands and iridium(III) center. It is commonly used as a catalyst in various organic transformations, including C-H activation, hydrogenation, and cross-coupling reactions. The (Pentamethylcyclopentadienyl)iridium(III) chloride dimer has been extensively studied for its reactivity and its role in promoting selective and efficient chemical reactions. Its unique structural and electronic properties make it a valuable tool in synthetic chemistry and materials science.
View more+
1. Names and Identifiers
2. Properties
3. Use and Manufacturing
4. Safety and Handling
5. MSDS
6. Synthesis Route
7. Precursor and Product
8. Computed Properties
11. Realated Product Infomation

 EN
EN