yellowish-white powderChEBI: A beta-diketone that is acetylacetone (acac) in which both methyl groups have been replaced by phenyl groups. It is a minor constituent of the root extract of licorice (Glycyrrhiza glabra) and exhibits antimutagenic and antica
cer effects.
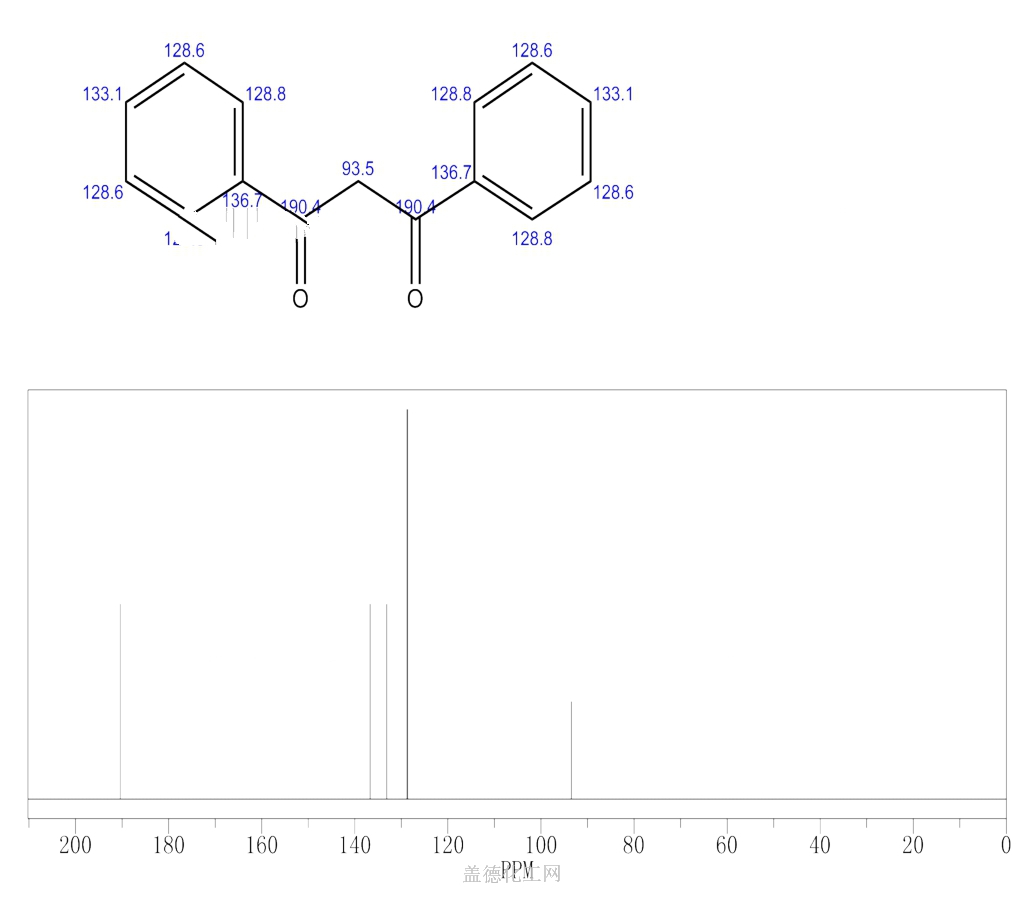
1,3-Diphenyl-1,3-propanedione (dibenzoylmethane, DBM) is an aromatic 1,3-diketone derivative of acetylacetone (acac), where both methyl groups in acac have been substituted by phenyl groups. It is a white solid melting at 77?78°C. Similar to acac, DBM exists in two tautomeric forms, with the keto-enol equilibrium of DBM shifting strongly towards the enol form, particularly in non-polar solvents such as benzene. This is the result of the stability of the intramolecular hydrogen bond in the cis-enol form which is further resonance-stabilized by conjugation with phenyl rings. Due to its high photostability, derivatives of DBM such as avobenzone, have found applications as sunscreen products.
DryPowder; DryPowder, PelletsLargeCrystals; OtherSolid
Dibenzoylmethane is a beta-diketone that is acetylacetone (acac) in which both methyl groups have been replaced by phenyl groups. It is a minor constituent of the root extract of licorice (Glycyrrhiza glabra) and exhibits antimutagenic and anticancer effects. It has a role as an antineoplastic agent, a metabolite and an antimutagen. It is a beta-diketone and an aromatic ketone.

 EN
EN

 Xi
Xi